 Now and Zen: A 1988 studio album recorded by Robert Plant, with guest contributions from Jimmy Page. [e] Now and Zen: A 1988 studio album recorded by Robert Plant, with guest contributions from Jimmy Page. [e]
| This article may be deleted soon.
|
|
|
|
|
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
| Now and Zen
|
| Image:NZalbum1988.jpg
|
| Type
|
Studio album
|
| Artist
|
Robert Plant
|
| Release Date
|
29 February 1988
|
| Recorded
|
October - November 1987 at
Swanyard Studios, London
Marcus Studios, London.
Mixed at Swanyard Studios, London.
|
| Genre
|
Hard rock, rock
|
| Language
|
English
|
| Length
|
46 minutes 59 seconds
|
| Label
|
Es Paranza Records
|
| Catalogue
|
Es Paranza 90863-1 (US)
Es Paranza 790 863-1 (UK)
|
| Producer
|
Robert Plant, Tim Palmer, and Phil Johnstone
|
| Engineer
|
Rob Bozas & Martin Russell
|
Now and Zen is an album by the former Led Zeppelin singer Robert Plant, released in 1988 via the record label Es Paranza. The album generated two Mainstream Rock Tracks|mainstream rock hits, 'Heaven Knows' (Number 1 for six weeks) and 'Tall Cool One' (Number 1 for four weeks), and earned Plant his first solo multi-platinum honour with RIAA.[1]
Overview
With a new backing band and time to rethink the direction of his career, Plant returned in late 1987 with more of the material that had historically defined him in Led Zeppelin. Although Plant persisted in utilising computerised audio technology, in a comparable fashion to his anteceding solo issues, for this release Plant re-integrated blues-rock that had all but been relinquished on his 1985 release Shaken 'n' Stirred.[2] Plant, who often uses mysterious and mystical lyrics, composes some of his most coherent songs, and the manner in which the writing complements the melodic arrangements are partially responsible for the commercial success of Now and Zen. A prominent guitar and an exotic aural texture to the recordings also marked another transformation in Plant's sound, who now added Middle Eastern colouration in compositions like 'Heaven Knows'. This is a musical direction that he would eventually re-engage with in the mid-1990s with the Jimmy Page and Robert Plant project.
This album is also notable in that it marks his first collaboration with keyboardist Phil Johnstone, who would continue to play and write with Plant on subsequent albums, and song-writer producer Dave Barrett. Plant's lifelong loyalty to his favourite Association football|football team Wolverhampton Wanderers (The Wolves) is expressed in the form of wolf motifs on the front cover. The working title for this recording project was in fact Wolves. In another symbolic return to his past, Plant's feather from Led Zeppelin IV in encapsulated in a crystal, next to the wolf motifs. The charting singles 'Heaven Knows' and 'Tall Cool One' features Led Zeppelin guitarist Jimmy Page (On the liner notes, Page's participation on the recordings were signified with a ZoSo symbol)[3], underpinning a riff similar to the Yardbirds-era standard 'The Train Kept a-Rollinˈ'. In retort to the Beastie Boys' unauthorised sampling of Led Zeppelin songs on their 1986 album Licensed to Ill, Plant also sampled Led Zeppelin tracks ('Whole Lotta Love', 'Black Dog', 'The Ocean (song)|The Ocean', and 'Custard Pie') on 'Tall Cool One', furthermore singing lyrical refrains from 'When the Levee Breaks (Led Zeppelin song)|When the Levee Breaks'.[4] Plant reflects with 'White, Clean and Neat', a song evoking teen life in the mid-1950s, when the arrival of rock 'n' roll divided families and whole generations. 'Walking Towards Paradise' was initially a bonus track obtainable only on the CD version of the album. Rhino Entertainment eventually issued a remastered edition of the album, with additional tracks, on 3 April 2007.
Plant performed 'Heaven Knows', 'Tall Cool One', and 'Ship of Fools' at the Atlantic Records 40th Anniversary concert in 1988. 'Ship of Fools' was also used on the final two-hour episode of Miami Vice entitled 'Freefall'.
In an interview he gave to Uncut (magazine)|Uncut magazine in 2005, Plant commented:
| ‘
|
By the time Now and Zen came out in '89, it looked like I was big again. It was a Top 10 album on both sides of the Atlantic. But if I listen to it now, I can hear that a lot of the songs got lost in the technology of the time.[5]
|
’
|
Track list
| Album information
|
|
1988 Track listing:
- 'Heaven Knows' (Phil Johnstone, David Barrett) – 4:02
- 'Dance on My Own' (Robert Plant, Phil Johnstone, Robert Crash) – 4:31
- 'Tall Cool One' (Robert Plant, Phil Johnstone) – 4:37
- 'The Way I Feel' (Robert Plant, Phil Johnstone, Doug Boyle) – 5:39
- 'Helen of Troy' (Robert Plant, Phil Johnstone) - 5:03
- 'Billy's Revenge' (Robert Plant, Phil Johnstone) – 3:33
- 'Ship of Fools' (Robert Plant, Phil Johnstone) – 4:59
- 'Why' (Robert Plant, Robert Crash) – 4:12
- 'White, Clean and Neat' (Robert Plant, Phil Johnstone) – 5:28
|
Chart positions
Album
| Chart (1988)
|
Peak Position
|
| Norwegian Albums Chart[6]
|
12
|
| UK Albums Chart[7]
|
10
|
| Swedish Albums Chart[8]
|
18
|
| Canadian RPM Top 100 Chart[9]
|
4
|
| US Billboard The 200 Albums Chart[10]
|
6
|
| Australian ARIA Top 50 Albums Chart[11]
|
27
|
| German Albums Chart[12]
|
48
|
| New Zealand RIANZ Top 40 Albums Chart
|
7
|
Singles
| Year
|
Single
|
Chart
|
Position
|
| 1988
|
'Heaven Knows'
|
UK Singles Chart[13]
|
33
|
| 1988
|
'Heaven Knows'
|
US Billboard Hot Mainstream Rock Tracks Chart[14]
|
1
|
| 1988
|
'Heaven Knows'
|
Canadian RPM Top 100 Chart[15]
|
65
|
| 1988
|
'Tall Cool One'
|
US Billboard Hot Mainstream Rock Tracks Chart[16]
|
1
|
| 1988
|
'Tall Cool One'
|
UK Singles Chart[17]
|
87
|
| 1988
|
'Tall Cool One'
|
Australian ARIA Top 50 Singles Chart[18]
|
47
|
| 1988
|
'Tall Cool One'
|
US Billboard Hot 100 Singles Chart[19]
|
25
|
| 1988
|
'Tall Cool One'
|
Canadian RPM Top 100 Chart[20]
|
15
|
| 1988
|
'Tall Cool One'
|
US Cash Box Top 100 Singles Chart[21]
|
31
|
| 1988
|
'Ship of Fools'
|
US Billboard Hot Mainstream Rock Tracks Chart[22]
|
3
|
| 1988
|
'Ship of Fools'
|
US Billboard Hot 100 Singles Chart[23]
|
84
|
| 1988
|
'Dance on My Own'
|
US Billboard Hot Mainstream Rock Tracks Chart[24]
|
10
|
| 1989
|
'Walking Towards Paradise'
|
US Billboard Hot Mainstream Rock Tracks Chart[25]
|
39
|
Certifications
Album
| Country
|
Sales
|
Certification
|
| United States (RIAA)
|
3,000,000+
|
3× Multi-Platinum[26]
|
Credits
| Personnel
|
- Musicians:
- Robert Plant - vocals, producer
- Jimmy Page – lead guitar ('Heaven Knows' and 'Tall Cool One')
- Phil Johnstone - keyboards, producer
- Doug Boyle - guitar
- Phil Scragg - bass guitar
- Chris Blackwell - drums, percussion
- Additional musicians:
- David Barrett - programming, keyboards, engineer
- Robert Crash - programming
- Marie Pierre - backing vocals
- Toni Halliday - backing vocals
- Kirsty MacColl - backing vocals
- Jerry Wayne - background voice ('White, Clean and Neat')
- Production:
- Tim Palmer - producer
- Bob Bozas - engineer
- Martin Russell - engineer
- Jonathan Dee - engineer
- Michael Gregovich - engineer
- Tim Burrell - engineer
- Richard Evans - design, art direction
- Davies & Starr - photography
|
Notes
- ↑ Pesselnick, Jill (October 2001). "Certifications: Beasties Toasted in Latest Certifications". Billboard 113 (43): 54. ISSN 0006-2510. Retrieved on 5 June 2009.
- ↑ Daniels, Neil (2008). Robert Plant: Led Zeppelin, Jimmy Page & the Solo Years, 1st. Church Stretton, Shropshire: Independent Music Press, 122. ISBN 0-9552822-7-6.
- ↑ Case, George (2007). Jimmy Page: Magus, Musician, Man - An Unauthorized Biography. New York: Hal Leonard, 174. ISBN 1-4234-0407-1.
- ↑ Lewis, Dave (2004). Led Zeppelin: The Complete Guide to Their Music. London: Omnibus Press. ISBN 1-84449-141-2.
- ↑ Williamson, Nigel. 'Good Times...Bad Times', Uncut (magazine)|Uncut, May 2005, p. 62.
- ↑ Top 40 Albums - 6 March 1988. norwegiancharts.com. Retrieved on 17 January 2009.
- ↑ Top 100 Albums - 12 March 1988. chartstats.com. Retrieved on 17 January 2009.
- ↑ Top 60 Albums - 16 March 1988. swedishcharts.com. Retrieved on 17 January 2009.
- ↑ RPM Albums Chart - 9 April 1988. RPM. Retrieved on 17 January 2009.
- ↑ The Billboard 200 - 21 May 1988. Billboard. Retrieved on 17 January 2009.
- ↑ Top 50 Albums - 3 July 1988. ARIA. Retrieved on 17 January 2009.
- ↑ Top 100 Albums - July 1988. charts-surfer.de. Retrieved on 19 January 2009.
- ↑ Top 100 Singles - 13 February 1988. chartstats.com. Retrieved on 19 January 2009.
- ↑ Hot Mainstream Rock Tracks - 20 February 1988. Billboard. Retrieved on 19 January 2009.
- ↑ RPM Singles Chart - 9 April 1988. RPM. Retrieved on 19 January 2009.
- ↑ Hot Mainstream Rock Tracks - 9 April 1988. Billboard. Retrieved on 19 January 2009.
- ↑ Top 100 Singles - 30 April 1988. chartstats.com. Retrieved on 19 January 2009.
- ↑ Top 50 Singles - 26 June 1988. ARIA. Retrieved on 19 January 2009.
- ↑ Hot 100 Singles - 2 July 1988. Billboard. Retrieved on 19 January 2009.
- ↑ RPM Singles Chart - 9 July 1988. RPM. Retrieved on 19 January 2009.
- ↑ Top 100 Singles - 9 July 1988. Cash Box. Retrieved on 19 January 2009.
- ↑ Hot Mainstream Rock Tracks - 11 June 1988. Billboard. Retrieved on 20 April 2009.
- ↑ Hot 100 Singles - 3 September 1988. Billboard. Retrieved on 19 January 2009.
- ↑ Hot Mainstream Rock Tracks - 13 August 1988. Billboard. Retrieved on 20 April 2009.
- ↑ Hot Mainstream Rock Tracks - 14 January 1989. Billboard. Retrieved on 20 April 2009.
- ↑ RIAA.org Now and Zen - 7 September 2001. RIAA. Retrieved on 20 April 2009.
|
Gareth Leng; Milton Beychok; Meg Ireland; Drew R. Smith
|
|
3
|
 Hawaiian alphabet: The form of writing used in the Hawaiian Language [e] Hawaiian alphabet: The form of writing used in the Hawaiian Language [e]
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
| Archive:New Draft of the Week
|
| Type
|
Alphabet
|
| Spoken languages
|
Hawaiian language
|
| Created by
|
American Protestant missionaries
|
| Time period
|
1822-Present
|
| Parent systems
|
→ Archive:New Draft of the Week
|
As an oral tradition, handed down generation after generation, the true origins of the Hawaiian language are relatively unknown. The Hawaiian alphabet, ka pī‘āpā Hawai‘ i, however, does not have such an obscure past. It was originally designed in the early 1800s by American missionaries who wanted to print a Hawaiian bible. Due to the language being passed down as an oral tradition, the missionaries had to adapt the Roman alphabet to fit their needs.
Origins
In 1778, British explorer James Cook made the first reported European discovery of Hawaiʻi. In his report, he wrote the name of the islands as "Owhyhee" or "Owhyee". By July 1823, they had begun using the phrase "Hawaiian Language." The actual writing system was developed by American Protestant missionaries on January 7, 1822. The original alphabet included
A, B, D, E, F, G, H, I, K, L, M, N, O, P, R, S, T, U, V, W, Y, Z
and seven diphthongs
AE, AI, AO, AU, EI, EU, OU.
In 1826, the developers voted to eliminate some of the letters which represented functionally redundant interchangeable letters, enabling the Hawaiian alphabet to approach the ideal state of one-symbol-one-sound, and thereby optimizing the ease with which people could teach and learn the reading and writing of Hawaiian.
- Interchangeable B/P. B was dropped, P was kept.
- Interchangeable L/R. L was kept, R was dropped.
- Interchangeable K/T. K was kept, T was dropped.
- Interchangeable V/W. V was dropped, W was kept.
Due to words with different meanings being spelled alike, use of the glottal stop became necessary. As early as 1823, the missionaries made limited use of the apostrophe to represent the glottal stop, but they did not make it a letter of the alphabet. In publishing the Hawaiian bible, they used the ʻokina to distinguish koʻu ('my') from kou ('your'). It wasn’t until 1864 that the ʻokina became a recognized letter of the Hawaiian alphabet.
Kahakō
As early as 1821, one of the missionaries, Hiram Bingham, was using macrons in making handwritten transcriptions of Hawaiian vowels. The macron, or kahakō, was used to differentiate between short and long vowels. The macron itself never became an official letter. Instead, a second set of vowels with macrons were added to the language as separate letters.
Modern Alphabet
 A children's alphabet book in Hawaiian Pronunciation
| Character |
Character Name |
IPA
|
| Aa |
'ā |
/a/
|
| Ee |
'ē |
/e/
|
| Ii |
'ī |
/i/
|
| Oo |
'ō |
/o/
|
| Uu |
'ū |
/u/
|
| Āā |
'ākō |
/aː/
|
| Ēē |
'ēkō |
/eː/
|
| Īī |
'īkō |
/iː/
|
| Ōō |
'ōkō |
/oː/
|
| Ūū |
'ūkō |
/uː/
|
| Hh |
hē |
/h/
|
| Kk |
kē |
/k/
|
| Ll |
lā |
/l/
|
| Mm |
mō |
/m/
|
| Nn |
nō |
/n/
|
| Pp |
pē |
/p/
|
| Ww |
wē |
/ʋ/
|
| ʻ |
ʻokina |
/ʔ/
|
Diphthongs
| Diphthongs
|
| Diphthongs |
Pronunciation |
Examples
|
| ai
|
i in ice
|
kai = sea water
|
| ae
|
I or eye
|
Maeʻola = Never-fading
|
| ao
|
ow in how
without nasal twang
|
Maoli = True
Kaona = Hidden Meaning
|
| au
|
ou in house or out
without nasal twang
|
Au = I, I am
|
| ei
|
ei in chow mein
or in eight
|
Lei = Garland
|
| eu
|
eh-(y)oo
|
ʻEleu = Lively
|
| iu
|
ee-(y)oo
similar to ew in few
|
Wēkiu = Topmost
|
| oe
|
oh-(w)eh
|
ʻOe = You
|
| oi
|
oi in voice
|
Poi = Hawaiian Staple
|
| ou
|
ow in bowl
|
Kou = your
|
| ui
|
oo-(w)ee in gooey
|
Hui = Together, team, Chorus
|
References
Alternative Hawaii
Omniglot (Read more...)
|
Drew R. Smith; Shamira Gelbam
|
|
2
|
 Air Quality Index: A number used by government agencies to characterize the quality of the ambient air at a given location. [e] Air Quality Index: A number used by government agencies to characterize the quality of the ambient air at a given location. [e]
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
 (CC) Photo: The Port of Los Angeles (California, USA) An air quality monitoring station. The Air Quality Index (AQI), also known as the Air Pollution Index (API), Pollutant Standard Index (PSI) or Air Quality Health Index (AQHI), is a number used by government agencies to characterize the quality of the ambient air at a given location. As the AQI increases, the severity of probable adverse health effects increases as does the percentage of the population expected to be affected by the adverse health effects.
To compute the AQI requires an air pollutant concentration to be obtained from an air quality monitoring station. The method used to convert from air pollutant concentrations to AQIs varies for each air pollutant, and is different in different countries.
In many countries, air quality index values are divided into ranges, and each range is assigned a descriptor (i.e., a very few words describing the air quality or the health effects of the range) and often a color code as well. A government agency might also encourage members of the public to avoid strenuous activities, use public transportation rather than personal automobiles and work from home when AQI levels are high.
Many countries monitor ground-level ozone, particulate matter (PM10), sulfur dioxide (S02), carbon monoxide (CO) and nitrogen dioxide (NO2) and calculate air quality indices for these pollutants. Most other air contaminants do not have an associated AQI.
Air Quality Indices by country
Canada's AQHI[1]
Air Quality
Health Index
(AQHI) |
Health Risk
Category |
Color
Code
|
| 1 – 3 |
Low |

|
| 4 – 6 |
Moderate |

|
| 7 – 10 |
High |

|
| 10+ |
Very High |

|
|
Canada
Environment Canada, the national environmental protection agency of Canada, uses Air Quality Health Index (AQHI) categories ranging from 1 to 10+ and each category has an assigned color code (see adjacent table) that enables members of the general public to easily identify their health risks as indicated in published air quality forecasts.[1]
As shown in the adjacent table:
- The three AQHI levels of 1, 2 and 3 are all in the low risk category.
- The three AQHI levels of 4, 5 and 6 are all in the moderate risk category.
- The four AQHI levels of 7, 8, 9 and 10 are all in the high risk category.
- The AQHI level of 10+ is the very high risk category.
As of 2009, many of the Canadian provinces, if not all, have adopted the AQHI categories implemented by Environment Canada.
China
China's National API[2]
Air Pollution
Index
(API) |
Air Quality
Level |
Air Quality
Category
|
| 0 – 50 |
I |
Excellent
|
| 51 – 100 |
II |
Good
|
| 101 – 200 |
III |
Slightly polluted
|
| 201 – 300 |
IV |
Moderately polluted
|
| 301+ |
V |
Heavily polluted
|
| Beijing's API[2]
|
| 0 – 50 |
|
Good
|
| 51 – 100 |
|
Moderate
|
| 101 – 150 |
|
Unhealthy for
sensitive groups
|
| 151 – 200 |
|
Unhealthy
|
| 201 – 250 |
|
Very unhealthy
|
| 251 – 500 |
|
Hazardous
|
|
Hong Kong's API[3]
Air Pollution
Index
(API) |
Health Effect
Category |
Color
Code
|
| 0 – 25 |
Low |
|
| 26 – 50 |
Medium |
|
| 51 – 100 |
High |
|
| 101 – 200 |
Very High |
|
| 201 – 500 |
Severe |
|
|
China's Ministry of Environmental Protection (MEP)[2][4] is responsible for monitoring the level of air pollution in China.
As of August 2008, MEP monitors daily pollution level in its major cities and develops an Air Pollution Index (API) level that is based on the ambient air concentrations sulfur dioxide, nitrogen dioxide, particulate matter (PM10), carbon monoxide, and ozone as measured at monitoring stations in each of those major cities.[2][4]
The adjacent table presents China's national API scale, which is not color coded and uses a scale 0 to more than 300, divided into five ranges of air quality categorized as excellent, good, slightly polluted, heavily polluted and hazardous.
API Mechanics
An individual score is assigned to the level of each pollutant and the final API is the highest of those 5 scores. The pollutant concentrations are obtained quite differently. Sulfur dioxide, nitrogen dioxide and PM10 concentrations are obtained as daily averages. Carbon monoxide and ozone are more harmful and are obtained as an hourly averages. The final API value is calculated as a daily average.[2][4]
The scale for each pollutant is non-linear, as is the final daily API value. Thus, an API value of 100 does not mean it is twice the pollution of API at 50, nor does it mean it is twice as harmful.
Beijing's API
China's capitol city, Beijing, has its own API scale, which was developed by the Beijing Municipal Environmental Protection Bureau.[5] As can be seen in the adjacent table, the API scale used by Beijing differs quite significantly from China's national scale in that:
• The Beijing scale ranges from 0 to 500 (rather than 0 to 300 as in the national scale)
• The Beijing scale is divided into six ranges of air quality (rather than five ranges as in the national scale).
Hong Kong
The Hong Kong Environmental Protection Department (Hong Kong EPD) has developed a color coded Air Pollution Index (API) based upon the measured concentrations of ambient particulate matter (PM10), sulfur dioxide, carbon monoxide, ozone and nitrogen dioxide over a 24-hour period.
Hong Kong's color coded Air Pollution Index (API) scale ranges from 0 to 500 corresponding to adverse health effects that range from low to severe as shown in the adjacent chart:[3]
- An API at or below 100 means that the pollutant levels are in the satisfactory range over 24 hour period and pose no acute or immediate health effects.
- Persistent high API values (51 to 100) in a year may mean that the annual Hong Kong Air Quality Objectives for protecting long-term health effects could be violated.
- API values in excess of 100 (very high) mean that levels of one or more pollutant(s) is/are in the unhealthy range. The Hong Kong EPD provides advice to the public regarding precautionary actions to take for such levels.
Although Hong Kong is now part of China, it can be seen that Hong Kong's API scale differs from both China's scale and Beijing's scale.
Malaysia's API[6]
Air Pollution
Index
(API) |
Air Quality
Category
|
| 0 – 50 |
Good
|
| 51 – 100 |
Moderate
|
| 101 – 200 |
Unhealthy
|
| 201 – 300 |
Very Unhealthy
|
| 301+ |
Hazardous
|
|
Malaysia
The air quality in Malaysia is described in terms of an Air Pollutant Index (API). The API is an indicator of air quality and was developed based on scientific assessment to indicate in an easily understood manner, the presence of pollutants and its impact on health. The API system of Malaysia closely follows the similar system developed by the U.S. Environmental Protection Agency (U.S. EPA). As shown in the adjacent table, Malaysia does not color code their air quality categories.
Monitoring stations measure the concentration of five major pollutants in the ambient air: PM10, sulfur dioxide, nitrogen dioxide, carbon monoxide and ozone. These concentrations are measured continuously on an hourly basis. The hourly value is then averaged over a 24-hour period for PM110 and sulfur dioxide and an 8-hour period for carbon monoxide. The ozone and nitrogen dioxide are read hourly. An hourly index is then calculated for each pollutant. The highest hourly index value is then taken as the API for the hour.
When the API exceeds 500, a state of emergency is declared in the reporting area. Usually, this means that non-essential government services are suspended, and all ports in the affected area closed. There may also be a prohibition on private sector commercial and industrial activities in the reporting area excluding the food sector.
Mexico's IMECA[7]
Air Quality
Index
(IMECA) |
Air Quality
Category |
Color
Code
|
| 0 – 50 |
Good |
|
| 51 – 100 |
Moderate |
|
| 101 – 200 |
Unhealthy |
|
| 201 – 300 |
Very Unhealthy |
|
| 301+ |
Extremely Unhealthy |
|
|
Mexico
The air quality in Mexico is described and reported hourly in terms of a color coded Metropolitan Index of Air Quality (IMECA), developed by the Ministry of the Environment for the Government of the Federal District.
The IMECA is calculated from the results of real-time monitoring of the ambient concentrations of ozone, sulfur dioxide, nitrogen dioxide, carbon monoxide and particulate matter (PM10).
The IMECA was developed specifically for the Federal District of Mexico which only encompasses Mexico City and its surrounding suburbs and adjacent municipalities.
The real-time monitoring of the ambient atmosphere is performed by the Sistema de Monitoreo Atmosférico de la Ciudad de México (SIMAT or System of Atmospheric Monitoring for Mexico City).
SIMAT's real-time monitoring includes monitoring of the ultra-violet (UV) radiation from the sun and the results are also described and reported hourly as IUVs (Índice de Radiación Ultravioleta) in a manner that is similar to the reporting of the IMECAs.[8]
Singapore's PSI[9]
Pollution
Standard Index
(24-hour PSI) |
Air Quality
Category
|
| 0 – 50 |
Good
|
| 51 – 100 |
Moderate
|
| 101 – 200 |
Unhealthy
|
| 201 – 300 |
Very Unhealthy
|
| 301+ |
Hazardous
|
|
Singapore
Singapore's National Environment Agency (NEA) in the Ministry of the Environment and Water Resources (MEWR) has the responsibility for the real-time monitoring of the concentrations of sulfur dioxide, nitrogen dioxide, carbon monoxide, ozone and PM10 in the ambient air of Singapore.
The real-time monitoring of the ambient air quality is done by a telemetric network of air quality monitoring stations strategically located in different parts of Singapore.
The NEA uses the real-time monitoring data to obtain and report 24-hour Pollution Standard Index (PSI) levels along with their corresponding air quality categories as shown in the adjacent table and which does not use color coding.[9]
The NEA states that the PSI scale developed for use in Singapore is very similar to the scale developed and used by the U.S. Environmental Protection Agency. The NEA also further states that the National Ambient Air Quality Standards (NAAQS) developed by the U.S. Environmental Protection Agency are used to assess Singapore's air quality.
Although the adjacent table indicates that the NEA categorizes a 24-hour PSI level that is higher than 300 as being hazardous, the NEA also considers a 24-hour PSI level higher than 400 to be life-threatening to ill and elderly persons.[10]
United Kingdom's API[11]
Air Pollution
Index (API) |
Health Effect
Banding |
Color
Code
|
| 1 – 3 |
Good |
|
| 4 – 6 |
Moderate |
|
| 7 – 9 |
High |
|
| 10 |
Very High |
|
|
United Kingdom
AEA Technology, a British environmental consulting company, issues air quality forecasts for the United Kingdom (UK) on behalf of the Department for Environment, Food and Rural Affairs (Defra).[11] The scale used in the United Kingdom is an Air Pollution Index (API) with levels ranging from 1 to 10 as shown in the attached table and it is color coded.
The scale was thoroughly studied and approved by the United Kingdom's government advisory body, namely the "Committee on Medical Effects of Air Pollution Episodes" (COMEAP).[11]
The scale is based on continuous monitoring, in locations throughout the United Kingdom, of the ambient air for the concentrations of the major air pollutants, namely sulfur dioxide, nitrogen dioxide, ozone, carbon monoxide and PM10. The forecasts issued by AEA Technology are based on the prediction of air pollution index for the worst-case of the five pollutants.
As shown in the adjacent table, the health effect of each API range is referred to as its banding rather than as its category. The health effect bandings for the API ranges are low, moderate, high and very high.
United States' AQI[12]
Air Quality
Index
(AQI) |
Air Quality
Category |
Color
Code
|
| 0 – 50 |
Good |
|
| 51 – 100 |
Moderate |
|
| 101 – 150 |
Unhealthy for
Sensitive Groups |
|
| 151 – 200 |
Unhealthy |
|
| 201 – 300 |
Very Unhealthy |
|
| 301 – 500 |
Hazardous |
|
|
United States
The Air Quality Index (AQI) ranges used by the U.S. Environmental Protection Agency (U.S. EPA) and their corresponding health effect categories and color codes are provided in the adjacent table. The U.S. EPA's AQI is also known as the Pollution Standards Index (PSI).
If multiple pollutants are measured at a monitoring site, then the largest or "dominant" AQI value is reported for the location.
The U.S. EPA has developed conversion calculators, available online,[13][14] for the conversion of AQI values to concentration values and for the reverse conversion of concentrations to AQI values.
A national map of the United States of America containing daily AQI forecasts across the nation, developed jointly by the U.S. EPA and NOAA is also available online.[15]
The U.S. Clean Air Act requires the U.S. EPA to review its National Ambient Air Quality Standards[16] every five years to reflect evolving health effects information. The Air Quality Index is adjusted periodically to reflect these changes.
Air pollutant concentration measurement units
In the United States, the concentrations of the air pollutants involved in the AQI are usually expressed as:
- Ozone and sulfur dioxides: ppbv = parts per billion (10 9) by volume = volume of pollutant gas per billion volumes of ambient air
- Carbon monoxide: ppmv = parts per million (10 6) by volume = volume of pollutant gas per million volumes of ambient air
- PM10, defined as particulate matter having an aerodynamic diameter of 10 μm (micrometer) or less: ug/m³ = micrograms of particulate matter per cubic metre of ambient air
- PM2.5, defined as particulate matter having an aerodynamic diameter of 2.5 μm (micrometer) or less: ug/m³ = micrograms of particulate matter per cubic metre of ambient air
References
(Read more...)
|
Paul Wormer; Milton Beychok
|
|
2
|
 Continuum hypothesis: A statement about the size of the continuum, i.e., the number of elements in the set of real numbers. [e] Continuum hypothesis: A statement about the size of the continuum, i.e., the number of elements in the set of real numbers. [e]
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
In mathematics, the continuum hypothesis is the statement that
any arbitrary infinite set of real numbers has either as many elements as there are real numbers or
only as many elements as there are natural numbers (i.e., there is no intermediate size).
This is equivalent to the statement
that there are as many real numbers as there are elements in the smallest set
which is larger than the set of natural numbers.
Since the set of real numbers (or the real line) is also called the continuum
this can be shortly expressed as:
Any set of real numbers is either countable or equivalent to the continuum.
This statement was first made by Georg Cantor (1877) when he studied subsets of the real line.
Cantor (who introduced sets and cardinal numbers) believed this to be true, but tried in vain to prove it.
From then on it stayed, for a long time, a prominent open mathematical problem to resolve.
In 1900, David Hilbert included the continuum hypothesis as the first problem,
therefore also called "first Hilbert problem",
in his famous lecture on 23 problems for the twentieth century.
The first step towards a solution was done in 1938 by Kurt Gödel
who showed that – in set theory including the axiom of choice –
the (generalized) continuum hypothesis cannot be proved to be false
(and thus is consistent with it).
Only much later, in 1963, Paul J. Cohen showed that it cannot be proved, either.
Hence the continuum hypothesis is independent of the usual (ZFC) axioms of set theory.
It therefore constitutes an important, not artificially constructed, example
for Gödel's Second Incompleteness Theorem.
Consequently, either the continuum hypothesis or, alternatively,
some contradicting assumption could be added to the axioms of set theory.
But since – in contrast to the situation with the axiom of choice –
there is no heuristically convincing reason to choose one of these possibilities,
the "working" mathematician usually makes no use of the continuum hypotheses,
and if a result depends on it, then it is explicitly mentioned.
Of course, in axiomatic set theory, and especially in the theory of cardinal and ordinal numbers,
the situation is different
and the consequences of the various choices concerning the continuum hypothesis are extensively studied.
The generalized continuum hypothesis is a much stronger statement
involving the initial sequence of transfinite cardinal numbers,
and is also independent of ZFC.
In terms of the arithmetic of cardinal numbers (as introduced by Cantor) the continuum hypothesis reads

while the generalized continuum hypothesis is

Georg Cantor 1877
The continuum hypothesis appears in a memoir of Cantor (dated Halle a.S., 11th July 1877, and published 1878)
in which he investigates sets of real numbers. He concludes with the following remark:
- Darnach würden die linearen Mannigfaltigkiten aus zwei Klassen bestehen von denen die erste alle Mannigfaltigkeiten in sich fasst, welche sich auf die Form: functio ips. ν (wo ν alle positiven ganzen Zahlen durchläuft) bringen lassen; während die zweite Klasse alle diejenigen Mannigfaltigkeiten in sich aufnimmt, welche auf die Form: functio ips. x (wo x alle reellen Werthe ≥0 und ≤1 annehmen kann) zurückführbar sind. Entsprechend diesen beiden Klassen würden daher bei unendlichen linearen Mannigfaltigkeiten nur zweierlei Mächtigkeiten vorkommen; die genaue Untersuchung dieser Frage verschieben wir auf eine spätere Gelegenheit.
Translated freely, this paragraph reads as follows:
- Hence the linear manifolds would consist of two classes of which the first contains all manifolds that can be written in the form: function of ν (where ν takes all positive integers); while the second class contains all those manifolds that have the form: function of x (where x takes all values ≥0 and ≤1). Hence, corresponding to these two classes, there would be only two cardinalities of infinite linear manifolds; the detailed investigation of this problem will be postponed on a later opportunity.
David Hilbert 1900
In his lecture on Mathematical problems,
delivered before the International Congress of Mathematicians at Paris in 1900,
David Hilbert states the continuum hypothesis as follows:
- 1. Cantors Problem von der Mächtigkeit des Continuums.
Zwei Systeme, d. h. zwei Mengen von gewöhnlichen reellen Zahlen (oder Punkten) heißen nach Cantor aequivalent oder von gleicher Mächtigkeit, wenn sie zu einander in eine derartige Beziehung gebracht werden können, daß einer jeden Zahl der einen Menge eine und nur eine bestimmte Zahl der anderen Menge entspricht. Die Untersuchungen von Cantor über solche Punktmengen machen einen Satz sehr wahrscheinlich, dessen Beweis jedoch trotz eifrigster Bemühungen bisher noch Niemanden gelungen ist; dieser Satz lautet:
Jedes System von unendlich vielen reellen Zahlen d. h. jede unendliche Zahlen- (oder Punkt)menge ist entweder der Menge der ganzen natürlichen Zahlen 1, 2, 3, ... oder der Menge sämmtlicher reellen Zahlen und mithin dem Continuum, d. h. etwa den Punkten einer Strecke aequivalent; im Sinne der Aeqivalenz giebt es hiernach nur zwei Zahlenmengen, die abzählbare Menge und das Continuum.
Aus diesem Satz würde zugleich folgen, daß das Continuum die nächste Mächtigkeit über die Mächtigkeit der abzählbaren Mengen hinaus bildet; der Beweis dieses Satzes würde mithin eine neue Brücke schlagen zwischen der abzählbaren Menge und dem Continuum.
In the English translation which was published in 1902:
- 1. Cantor's problem of the cardinal number of the continuum
Two systems, i. e., two assemblages of ordinary real numbers or points, are said to be (according to Cantor) equivalent or of equal cardinal number, if they can be brought into a relation to one another such that to every number of the one assemblage corresponds one and only one definite number of the other. The investigations of Cantor on such assemblages of points suggest a very plausible theorem, which nevertheless, in spite of the most strenuous efforts, no one has succeeded in proving. This is the theorem:
Every system of infinitely many real numbers, i. e., every assemblage of numbers (or points), is either equivalent to the assemblage of natural integers, 1, 2, 3,... or to the assemblage of all real numbers and therefore to the continuum, that is, to the points of a line; as regards equivalence there are, therefore, only two assemblages of numbers, the countable assemblage and the continuum.
From this theorem it would follow at once that the continuum has the next cardinal number beyond that of the countable assemblage; the proof of this theorem would, therefore, form a new bridge between the countable assemblage and the continuum.
Hilbert continues this problem, now known as the "First Hilbert Problem" by describing another unproven claim of Cantor
(which he thought to likely be related), namely
the statement that there is a well-order of the real numbers.
This property, however, turned out to be a consequence of the axiom of choice.
Kurt Gödel 1947
In an essay (published 1947, after his proof and before Cohen's result) Kurt Gödel
argued that even if the continuum hypothesis would turn out to be independent (as he expected)
this would not imply that it cannot be solved at all:
- There might exist axioms so abundant in their verifiable consequences, shedding so much light upon a whole discipline, and furnishing such powerful methods for solving given problems (and even solving them, as far as that is possible, in a constructivistic way) that quite irrespective of their intrinsic necessity they would have to be assumed at least in the same sense as any well established physical theory.
He continues with a discussion of several arguments which support his position
that the continuum hypothesis is likely to be wrong. (Read more...)
|
Milton Beychok; Howard C. Berkowitz
|
|
2
|
 Edward Teller: (1908-2003) One of the most controversial scientists of the 20th century because of his role as the main developer of the hydrogen bomb, his outspoken defense of an unassailable nuclear arsenal, and support for President Reagan's Strategic Defensive Initiative. [e] Edward Teller: (1908-2003) One of the most controversial scientists of the 20th century because of his role as the main developer of the hydrogen bomb, his outspoken defense of an unassailable nuclear arsenal, and support for President Reagan's Strategic Defensive Initiative. [e]
Edward Teller (1908–2003) was an eminent and controversial theoretical physicist. He was born as Teller Ede in Budapest (Hungary) on January 15, 1908. He died in his home on the Stanford campus (Palo Alto, California) on September 9, 2003. He had been a senior research fellow at Stanford University's Hoover Institution since 1975 when he retired as professor of the University of California, Berkeley and as associate director of the Lawrence Livermore National Laboratory.
Edward Teller was one of the most controversial scientists of the 20th century because of his role as an advocate and conceptual designer of the hydrogen bomb, his outspoken defense of an unassailable nuclear arsenal, and support for President Reagan's Strategic Defensive Initiative ("Star Wars") ballistic missile defense program. During the McCarthy era he alienated many of his colleagues by his testimony in the 1954 security clearance hearings of J. Robert Oppenheimer, his former colleague and director of the Los Alamos Laboratory.
Youth
Edward Teller was born to Max Teller and Idona Deutsch, who both were assimilated Hungarian Jews. Edward's mother Idona was an accomplished pianist who gave up her aspirations to a concert career when she married Edward's father, who was a lawyer. As a young boy Edward experienced a short and fierce communist dictatorship under Béla Kun (March 21, 1919 – August 1, 1919); it has been suggested that his rabid aversion of communism in later life was rooted in this experience. The Hungarian communists were soon ousted by Rear Admiral Miklós Horthy who headed a fascist regime until the end of World War II.
In 1918 Edward entered the famous gymnasium "Minta" ("Model"; an advanced German type of high school founded by the father of Theodore von Kármán), where he met his later wife Augusta Maria ("Mici") Harkányi, who was a sister of one of Edward's closest school friends. The Harkányis were from Jewish descent but had converted to Calvinism. After finishing the gymnasium, Edward spent a few months at the university in Budapest, but January 2, 1926 he moved to Karlsruhe in Germany to study chemical engineering. Karlsruhe was at that time the seat of one of the most outstanding technical universities of the country; especially its chemical engineering was strong because of its close cooperation with I.G. Farben, in those days world's largest chemical company. In April 1928 Edward left the field of chemical engineering and moved to Munich to study theoretical physics under Arnold Sommerfeld, a great mathematical physicist who made important contributions to the development of quantum mechanics. (Read more...)
|
Milton Beychok; Howard C. Berkowitz
|
|
2
|
 William Harvey: (1579–1657) English physician who discovered the true nature of blood circulation and the function of the heart as a pump. [e] William Harvey: (1579–1657) English physician who discovered the true nature of blood circulation and the function of the heart as a pump. [e]
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
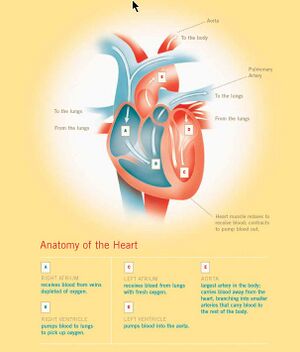 (PD) Image: Levine & Associates, Inc. for U.S National Institutes of Health, National Institute on Aging at: http://bit.ly/MnJaE Anatomy of the Human Heart. For enlarged version of this image showing more detail, click here. William Harvey (1578-1657) bestowed on humanity one of the most important advances in the history of medical science — an explanation of the core physiology of the human cardiovascular system. In part by introducing quantitative methods into anatomical and physiological investigations, Harvey discovered that the left ventricle of the heart pumps blood through the body, doing so via a system of vessels such that the blood moves in a circular path,[1] from the left side of the heart through the arteries and back to the right side of the heart through the veins, transiting from the right side of the heart to the left via blood vessels in the lung, the two sides of the heart separated by a blood-impermeable septum. He published those findings in his 1628 book, Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus (Anatomical Exercises on the Motion of the Heart and Blood in Animals), usually referred to as De Motu Cordis.[2] [3] [4]
In his dissections of humans and animals, Harvey could not see vessels connecting the arteries to the veins, since, as it turns out, their minute size lies below the limits of visual acuity, even with the magnifying glass he used in his work. He had no access, and perhaps no knowledge, of the existence of microscopes, however primitive their state. Harvey could only infer that a connecting pathway existed. In 1661, a few years after Harvey died, the Italian biologist, Marcello Malpighi (1628-1694), using one of the early microscopes, discovered capillaries, tiny blood vessels not visible to the naked eye, connecting arteries to veins. In a seemingly fitting coincidence, Malpighi had entered the world the same year Harvey published De Motu Cordis.
In works published little more than a century apart, 1543 to 1661, three men, Andreas Vesalius (1514-1564), William Harvey (1578-1657), and Marcello Malpighi (1628-1694), demonstrated central truths of human anatomy and physiology that had escaped Western medicine for more than a millennium following the erroneous teachings of the influential Greek physician, Galen of Pergamum (130-216 CE). It required three investigators to break the stranglehold of one.
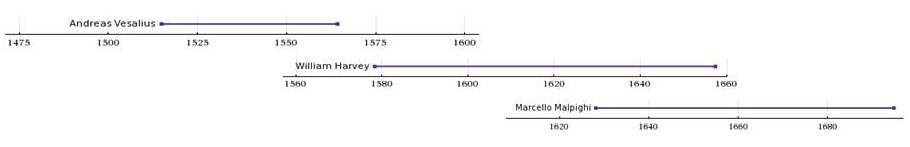 (CC) Image: Anthony Sebastian (adapted from data from Wolfram Alpha)
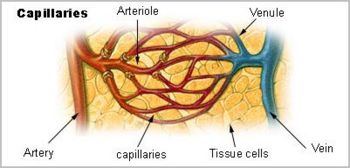 Courtesy U.S. Cancer Institute. Cartoon showing capillaries, visible only with a microscope, connecting macroscopically visible arteries and veins. William Harvey’s Major Contributions
Adapted from Sherwin B. Nuland (2008)[5]
- He overturned the erroneous view of the roles of the heart, arteries and veins that Galen had taught — by delineating the cardinal features of the map depicting the transport path of blood through the body — that blood circulates continuously through the body from the heart and back to the heart; Harvey writes:
And now returning to my immediate subject, I go on with what yet remains for demonstration, viz., that in the more perfect and warmer adult animals, and man, the blood passes from the right ventricle of the heart by the pulmonary artery, into the lungs, and thence by the pulmonary veins into the left auricle, and from there into the left ventricle of the heart. And, first, I shall show that this may be so, and then I shall prove that it is so in fact. De Motus Cordis, Chapter VI: Of The Course By Which the Blood Is Carried
First of all, the auricle contracts, and in the course of its contraction forces the blood (which it contains in ample quantity as the head of the veins, the store-house and cistern of the blood) into the ventricle, which, being filled, the heart raises itself straightway, makes all its fibres tense, contracts the ventricles, and performs a beat, by which beat it immediately sends the blood supplied to it by the auricle into the arteries. The right ventricle sends its charge into the lungs by the vessel which is called vena arteriosa, but which in structure and function, and all other respects, is an artery. The left ventricle sends its charge into the aorta, and through this by the arteries to the body at large. De Motu Cordis, Chapter V: Of The Motion, Action And Office Of The Heart
- He reintroduced the concept and practice of experimentation in medical studies, earlier introduced by Galen but mostly ignored by medical researchers for nearly one and a half millennia;
- He introduced the use of quantitative methods in medical research — by estimating the volume of blood pumped by the heart each day and arguing the improbability that the body could generate that amount each day, even if it converted to blood everything ingested each day. Psychologist and science journalist, Robert E. Adler, gives a elegant description:
The breakthrough came when Harvey started to think in terms of numbers—for the first time in the history of medicine. He knew that the left ventricle of the human heart expelled about two ounces of blood with each contraction. If the heart beat 72 times a minute, in one hour it would pump 540 pounds of blood—three times the weight of a grown man. With that simple calculation he ended two millennia of speculation. The liver could not possibly generate more than a person's entire weight in blood in an hour. And however it was generated or utilized, that much blood could not be on a one-way trip to the periphery of the body. So much blood could be moving through the body only if it was being recycled. "I began to think there was a sort of motion as in a circle," he wrote, in what now sounds like a thunderous understatement.[6]
- Harvey introduced his quantitation method in De Motu Cordis with these words:
Thus far I have spoken of the passage of the blood from the veins into the arteries, and of the manner in which it is transmitted and distributed by the action of the heart; points to which some, moved either by the authority of Galen or Columbus, or the reasonings of others, will give in their adhesion. But what remains to be said upon the quantity and source of the blood which thus passes is of a character so novel and unheard-of that I not only fear injury to myself from the envy of a few, but I tremble lest I have mankind at large for my enemies, so much doth wont and custom become a second nature. Doctrine once sown strikes deep its root, and respect for antiquity influences all men. Still the die is cast, and my trust is in my love of truth and the candour of cultivated minds. Chapter VIII: Of The Quantity Of Blood Passing Through The Heart
- Independently of his contemporary, Francis Bacon, he showed that reasoning by induction — generalizing from a collection of separate but related facts — could yield valid inferences about human physiology. Harvey writes:
Since all things, both argument and ocular demonstration, show that the blood passes through the lungs, and heart by the force of the ventricles, and is sent for distribution to all parts of the body, where it makes its way into the veins and porosities of the flesh, and then flows by the veins from the circumference on every side to the centre, from the lesser to the greater veins, and is by them finally discharged into the vena cava and right auricle of the heart, and this in such a quantity or in such a flux and reflux thither by the arteries, hither by the veins, as cannot possibly be supplied by the ingesta, and is much greater than can be required for mere purposes of nutrition; it is absolutely necessary to conclude that the blood in the animal body is impelled in a circle, and is in a state of ceaseless motion; that this is the act or function which the heart performs by means of its pulse; and that it is the sole and only end of the motion and contraction of the heart. De Motu Cordis, Chapter XIV: Conclusion of the Demonstration of the Circulation
Brief sketch of Williams Harvey’s life
Born in 1578 (April 1, at Folkstone, on the east coast of Kent, England), of Thomas and Joan Harvey, as the eldest of seven brothers and two sisters (a "week of brothers" and a "brace of sisters"), William Harvey entered the world shortly after Andreas Vesalius (1514-1564) had died, though Vesalius's reputation had not died, owing to his remarkably detailed and elegantly drawn illustrations revolutionizing the understanding of human anatomy.[7] [8] [9] For his anatomical work, William Harvey had Vesalius's giant shoulders to stand on, and ultimately he saw further.
Harvey received his early education in the classics, in Canterbury, at King's School (1588-1593), there "....admonished to speak Greek or Latin even on the playground." [6] Harvey's father, a landowner and successful merchant, could afford to send Harvey to the University of Cambridge (specifically, Gonville and Caius College), which he entered at age 16 years (1593) and received his Bachelor of Arts (B.A.) degree at age 19 years (1597). Harvey developed an interest in medicine and decided to go to Italy, one of the major centers of intellectual activity in Europe at the time. He enrolled in the then renown University of Padua, studying medicine under Hieronymus Fabricius of Aquapendente, a noted anatomist in the Vesalian tradition, who had discovered the valves in the veins, a discovery which later contributed to Harvey's thinking that led to his discovery of the blood circulatory system.[10] Harvey's earlier education in the classics helped ease his learning at Padua, as lecturers spoke in Latin. Harvey received his Doctor of Medicine degree in April, 1602, at age 24 years.[11]
After Padua, Harvey returned to England and developed a practice in medicine, married, and became a Fellow of the College of Physicians in London. He also secured a position as physician at St. Bartholomew’s Hospital, one of London’s great hospitals, and there and in his private practice distinguished himself as a physician. In 1615, at age 37 years, the College of Physicians elected him their Professor of Anatomy and Surgery, and gave him the honor of the Lumleian Lectureship, a lifetime remunerated position, in which he lectured on human anatomy, physiology and surgery, including performing demonstration dissections on human corpses, officially twice per week, from 1616 to 1656, the year before he died. The lecturership gave Harvey a great opportunity to organize his thinking and guide his research. His lecture notes survive as Lectures on the Whole of Anatomy as a manuscript in the British Library and in English translation.[12]
It is from the fabric of these short notes on the heart that De Motu Cordis was to be built. From 1616 until after the publication of De Motu Cordis there is no contemporary account of the impact of Harvey's lectures on his audience. However, in the Royal College of Physicians there is a manuscript of the anatomical lectures given by Baldwin Hamey (1600-1676), one of the great benefactors of the College, on January 22, 24, and 25, 1647/48. The manuscript, in Hamey's hand, gives us an excellent picture not only of the author's learning but also of the full content of lectures of this type. He had attended Harvey's lectures and obviously profited from them. When discussing the movement of the blood, Hamey gives a discussion on the theories put forward by the ancients and his predecessors and then says:[13]
From the left ventricle, it is, as I sayd, expel'd by the arteries into the whole body; and there, till of late yeares, it was thought to rest, nor was there any further heed taken, or account given of it, than this; that it served for nutrition and augmentation, for generation of spirits and of sperme in their due times. But now by the conduct of our renowned Professor and Colleague Dr. Harvey, there is a way found to bringe the greatest part back againe, and yet no part of the foresaid worke left undone. So that now we truely know what . . . is meant by Circularis Disciplina, for it may be shew'd us in every body that hath a Heart.
In 1618 Harvey became physician extraordinary to the king (James I), and ministered to many eminent aristocrats, including Francis Bacon, for whom he had little regard as an intellectual. After Charles I succeeded the throne, in 1625, Harvey became Charles' physician, benefitting from the King’s patronage to pursue his medical investigations. When civil strife engulfed England, Harvey, now in his 60s retired to live with a brother, pursuing his experiments until he died in 1657, having lived nearly to the age of 80 years.[14] [15]
De Motu Cordis
To read the full-text of De Motu Cordis in English translation, click on the "Works" tab in the banner at the beginning of this page. Equivalently, click De Motus Cordis, which brings you to same subpage of this article. A few revelatory quotes from the work:
True philosophers, who are only eager for truth and knowledge, never regard themselves as already so thoroughly informed, but that they welcome further information from whomsoever and from wheresoever it may come; nor are they so narrow-minded as to imagine any of the arts or sciences transmitted to us by the ancients, in such a state of forwardness or completeness, that nothing is left for the ingenuity and industry of others.
And no one denies the blood as such, even the portion of it which flows in the veins, is imbued with spirits. But if that portion of it which is contained in the arteries be richer in spirits, it is still to be believed that these spirits are inseparable from the blood, like those in the veins; that the blood and spirits constitute one body (like whey and butter in milk, or heat in hot water), with which the arteries are charged, and for the distribution of which from the heart they are provided.
Of course, we now know that the richer 'spirit' in arterial blood is oxygen. Before the discovery of oxygen, the alchemists of the seventeenth century recognized that air contained an essential ingredient, an 'elixir of life' — a kind of 'spirit'. We also know today that venous blood, too, has its richer 'spirit', carbon dioxide (as bicarbonate).
De Generatione Animalium
Harvey received less repute for his other great work, De Generatione Animalium — On the Generation of Animals — a contribution to embryology....
References cited and notes
- ↑ Note: Not 'circular' in the strict geometrical sense of all points on the path equidistant from a center, but in the sense of a closed curve of irregular shape though with end-point and start-point at the same place.
- ↑ Harvey W. (1628) On the Motion of the Heart and Blood in Animals. Translation: Robert Willis. The Internet Modern History Sourcebook. Paul Halsall, halsall@fordham.edu, Sourcebook Compiler.
- ↑ Note: Read the full-text of Robert Wills' English translation of De Motu Cordis in this Citizendium article's subpage, Works (click Works tab at top of this page, or click here).
- ↑ Harvey W. (1628) EXERCITATIO ANATOMICA DE MOTU CORDIS ET SANGUINIS IN ANIMALIBUS. Facsimile of original, with English translation and Annotations by Chauncey D. Leake, Professor of Pharmacology, University of California San Francisco. Tercentennial Edition. Charles C. Thomas: Springfield IL. Full-Text viewable online or via free PDF download. Courtesy Biodiversity Heritage Library.
- ↑ Nuland SB. (2008) Doctors: The History of Scientific Medicine Revealed Through Biography. The Teaching Company. (12 lectures, 30 minutes/lecture), Course No. 8128.
- ↑ 6.0 6.1 Adler RE. (2004) Medical Firsts: From Hippocrates to the Human Genome. Hoboken NJ: Wiley.
- ↑ The Galileo Project: Harvey, William
- Note: Scholarly summary of William Harvey's life and work, extensively referenced.
- ↑ William Harvey (2008) Encyclopedia Britannica Online Free Full-Text Article edited by British physician, surgeon, medical historian and bibliophile, Sir Geoffrey Langdon Keynes
- ↑ Sir D´Arcy Power. (1897) William Harvey [Free full-text Google book. T. Fisher Unwin:London
- ↑ Note: Fabricius did not call them valves, but 'little doors', not completely closed doors, closed just enough to slow down the blood flow as it flowed away from the heart, opposite of its true direction of flow but in the direction Galen had taught that it flowed.
- ↑ Note: Of interest, Galileo was Professor of Mathematics at the University of Padua when Harvey was a student there.
- ↑ Harvey W. (1961; originally written 1616-?) Lectures on the Whole of Anatomy: An Annotated Translation of Prelectiones Anatomiae Universalis. C. D. O'Malley - transltr, F. N. L. Poynter - transltr, K. F. Russell - transltr. University of California Press. Berkeley, CA.
- From the translators' Introduction, page 6: The circumstances attending the production of these lecture notes have never been discussed, but the more closely they are investigated the clearer does it become that many features of them which have been taken for granted are still open to question. They are certainly notes that Harvey prepared for his Lumleian lectures, and, judging by their scope, by the research into the literature which is revealed in the citations, and by the personal observations briefly referred to, Harvey must have spent much time in compiling them.
- ↑ Harvey W. (1961 edition) Lectures on the Whole of Anatomy: An Annotated Translation of Prelectiones Anatomiae Universalis. C.D. O´Malley, transltr; F. N. L. Poynter, transltr; K. F. Russell, transltr. University of California Press: Berkeley, CA. (From the translators' Introduction, page 17)
- ↑ Huxley TH. (1878) William Harvey and the Discovery of the Circulation of the Blood. (A free full-text PDF download) A Lecture delivered in the Free Trade Hall, November 2nd, 1878. From the Project Gutenberg Literary Archive Foundation [Etext #2939].
- ↑ William Harvey (1578-1657). Originally appearing in Volume V13, Page 47 of the 1911 Encyclopedia Britannica.
(Read more...)
|
|
Anthony Sebastian
|
3
|
 Heat: A form of energy that flows spontaneously from hotter to colder bodies that are in thermal contact. [e] Heat: A form of energy that flows spontaneously from hotter to colder bodies that are in thermal contact. [e]
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
 PD Image Energy of the hot gas flame flows into the kettle and the liquid water in it. Heat is a form of energy that is transferred between two bodies that are in thermal contact and have different temperatures. For instance, the bodies may be two compartments of a vessel separated by a heat-conducting wall and containing fluids of different temperatures on either side of the wall. Or one body may consist of hot radiating gas and the other may be a kettle with cold water, as shown in the picture. Heat flows spontaneously from the higher-temperature to the lower-temperature body. The effect of this transfer of energy usually, but not always, is an increase in the temperature of the colder body and a decrease in the temperature of the hotter body.
Change of aggregation state
A vessel containing a fluid may lose or gain energy without a change in temperature when the fluid changes from one aggregation state to another. For instance, a gas condensing to a liquid does this at a certain fixed temperature (the boiling point of the liquid) and releases condensation energy. When a vessel, containing a condensing gas, loses heat to a colder body, then, as long as there is still vapor left in it, its temperature remains constant at the boiling point of the liquid, even while it is losing heat to the colder body. In a similar way, when the colder body is a vessel containing a melting solid, its temperature will remain constant while it is receiving heat from a hotter body, as long as not all solid has been molten. Only after all of the solid has been molten and the heat transport continues, the temperature of the colder body (then containing only liquid) will rise.
For example, the temperature of the tap water in the kettle shown in the figure will rise quickly to the boiling point of water (100 °C). Then, when the flame is not switched off, the temperature inside the kettle remains constant at 100 °C for quite some time, even though heat keeps on flowing from flame to kettle. When all liquid water has evaporated—when the kettle has boiled dry—the temperature of the kettle will quickly rise again until it obtains the temperature of the burning gas, then the heat flow will finally stop. (Most likely, though, the handle and maybe the metal of the kettle, too, will have melted before that).
Units
At present the unit for the amount of heat is the same as for any form of energy. Before the equivalence of mechanical work and heat was clearly recognized, two units were used. The calorie was the amount of heat necessary to raise the temperature of one gram of water from 14.5 to 15.5 °C and the unit of mechanical work was basically defined by force times path length (in the old cgs system of units this is erg). Now there is one unit for all forms of energy, including heat. In the International System of Units (SI) it is the joule, but the British Thermal Unit and calorie are still occasionally used. The unit for the rate of heat transfer is the watt (J/s).
Equivalence of heat and work
Although heat and work are forms of energy that both obey the law of conservation of energy, they are not completely equivalent. Work can be completely converted into heat, but the converse is not true. When converting heat into work, part of the heat is not—and cannot be—converted to work, but flows to the body of lower temperature that is out of necessity present to generate a heat flow.
Heat and temperature
The important distinction between heat and temperature (heat being a form of energy and temperature a measure of the amount of that energy present in a body) was clarified by Count Rumford, James Prescott Joule, Julius Robert Mayer, Rudolf Clausius, and others during the late 18th and 19th centuries. Also it became clear by the work of these men that heat is not an invisible and weightless fluid, named caloric, as was thought by many 18th century scientists, but a form of motion. The molecules of the hotter body are (on the average) in more rapid motion than those of the colder body. The first law of thermodynamics, discovered around the middle of the 19th century, states that the (flow of) heat is a transfer of part of the internal energy of the bodies. In the case of ideal gases, internal energy consists only of kinetic energy and it is indeed only this motional energy that is transferred when heat is exchanged between two containers with ideal gases. In the case of non-ideal gases, liquids and solids, internal energy also contains the averaged inter-particle potential energy (attraction and repulsion between molecules), which depends on temperature. So, for non-ideal gases, liquids and solids, also potential energy is transferred when heat transfer occurs.
Forms of heat
The actual transport of heat may proceed by electromagnetic radiation (as an example one may think of an electric heater where usually heat is transferred to its surroundings by infrared radiation, or of a microwave oven where heat is given off to food by microwaves), conduction (for instance through a metal wall; metals conduct heat by the aid of their almost free electrons), and convection (for instance by air flow or water circulation).
Entropy
If two systems, 1 (cold) and 2 (hot), are isolated from the rest of the universe (i.e., no other heat flows than from 2 to 1 and no work is performed on the two systems) then the entropy Stot = S1 + S2 of the total system 1 + 2 increases upon the spontaneous flow of heat. This is in accordance with the second law of thermodynamics that states that spontaneous thermodynamic processes are associated with entropy increase.
In general, the entropy S of a system at absolute temperature T increases with

when it receives an amount of heat Q > 0. Entropy is an additive (size-extensive) property.
The hotter system 2 loses an amount of heat to the colder system 1. In absolute value the exchanged amounts of heat are the same by the law of conservation of energy (no energy escapes to the rest of the universe), hence

Here it is assumed that the amount of heat Q is so small that the temperatures of the two systems are constant. One can achieve this by considering a small time interval of heat exchange and/or very large systems.
Remark: the expression ΔS = Q/T is only strictly valid for a reversible (also known as quasistatic) flow of energy. It is possible[1] to define:

It is assumed that ΔSint is much smaller than ΔSext, so that it can be neglected.
Semantic caveats
It is strictly speaking not correct to say that a hot object "possesses much heat"—it is correct to say, however, that it possesses high internal energy. The word "heat" is reserved to describe the process of transfer of energy from a high temperature object to a lower temperature one (in short called "heating of the cold object"). The reason that the word "heat" is to be avoided for the internal energy of an object is that the latter can have been acquired either by heating or by work done on it (or by both). When we measure internal energy, there is no way of deciding how the object acquired it—by work or by heat. In the same way as one does not say that a hot object "possesses much work", one does not say that it "possesses much heat". Yet, terms as "heat reservoir" (a system of temperature higher than its environment that for all practical purposes is infinite) and "heat content" (a synonym for enthalpy) are commonly used and are incorrect by the same reasoning.
The molecules of a hot body are in agitated motion and, as said, it cannot be measured how they became agitated, by work or by heat. Often, especially outside physics, the random molecular motion is referred to as "thermal energy". In classical (phenomenological) thermodynamics this is an intuitive, but undefined, concept. In statistical thermodynamics, thermal energy could be defined (but rarely ever is) as the average kinetic energy of the molecules constituting the body. Kinetic and potential energy of molecules are concepts that are foreign to classical thermodynamics, which predates the general acceptance of the existence of molecules.
Quotation
As a result Carathéodory was able to obtain the laws of thermodynamics without recourse to fictitious machines or objectionable concepts as the flow of heat.[2]
Reference
- ↑ E. A. Guggenheim, Thermodynamics, 5th edition, North Holland (1967). p. 17
- ↑ H. Margenau and G. M. Murphy, The Mathematics of Physics and Chemistry, 2nd edition, Van Nostrand Company, New York (1956) p. 29
|
|
Joe Quick
|
1
|
Current Winner (to be selected and implemented by an Administrator)
To change, click edit and follow the instructions, or see documentation at {{Featured Article}}.
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right.
|
Do you see this on PREVIEW? then SAVE! before following a link.
A - For a New Cluster use the following directions
Subpages format requires a metadata page.
Using the following instructions will complete the process of creating this article's subpages.
- Click the blue "metadata template" link below to create the page.
- On the edit page that appears paste in the article's title across from "
pagename =".
- You might also fill out the checklist part of the form. Ignore the rest.
- For background, see Using the Subpages template Don't worry--you'll get the hang of it right away.
- Remember to hit Save!
 the "metadata template". the "metadata template".
However, you can create articles without subpages. Just delete the {{subpages}} template from the top of this page and this prompt will disappear. :) Don't feel obligated to use subpages, it's more important that you write sentences, which you can always do without writing fancy code.
|
B - For a Cluster Move use the following directions
The metadata template should be moved to the new name as the first step. Please revert this move and start by using the Move Cluster link at the top left of the talk page.
The name prior to this move can be found at the following link.
|
|
A wrench (American English), or spanner (British English), is a fastening tool used to manipulate threaded fasteners such as nuts, studs and bolts. They also may manipulate threaded structural elements such as pipes. The wrench is sized and shaped to put pressure and leverage on flat faces of the fastener, and then is moved in the direction of rotation needed to loosen or tighten the fastening assembly.
Hand tools
The most common hand wrenches are made either from a flat bar of steel, or from cylinders. Even with these two types, the wrench can either completely or partially surround the fastener.
Flat Bar
The most common hand wrenches either are open-ended, such that they have sides parallel to two or more facets of the nut, or box, which surrounds all sides of the nut. These types can drop over the nut no matter how much of the screw or bolt protrudes.
The longer the handle of the wrench, the more force can be applied to the fastener. One technique is to carry a length of pipe, which can slip over the wrench and act as a handle extender. Do be warned, however, that a sufficiently long pipe can exert enough force to break the wrench or the fastener.
 Double-sided open-end wrench  Double-sided box-end wrench Still, there are times when it is absolutely necessary to release a stuck fastener. It is usually wise first to apply a penetrating lubricant to the fastener, and tap it gently to let the lubricant reach the threads, waiting briefly before applying force. Yet another dangerous but sometimes necessary technique is to hit the handle of the wrench with a hammer; do remember to hit it in the direction in which the fastener needs to turn. Gloves and eye protection are wise precautions when hammering a wrench. An even more desperate expedient is to heat the fastener to expand it, which may destroy the hardness or toughness of the metal. Occasionally, it is possible to chill the piece to which the fastener is attached, contracting it and helping break the adhesion.
For some specialized applications, typically where only authorized persons are to adjust the fastener, the sides of the wrench head may not be parallel. For example, the nuts on fire hydrants are pentagonal, so they cannot be manipulated with a standard wrench shape.
Combination wrench
 (CC) Photo: Derek Hodges A combination wrench Adjustable open wrench
 PD Image Adjustable wrench An adjustable wrench has one fixed jaw, and one that is adjustable by means of a screw adjustment, usually made in one piece with the adjustable jaw. In the U.S., this is often called a Crescent wrench after the first well-known manufacturer, while it is a Bahco wrench in Dutch usage, after the Swedish manufacturer. The obvious advantage of the adjustable wrench is that an entire set of wrenches need not be carried when the size of the fastener to be adjusted is not known. The disadvantage is that the adjustment can slip, and generally cannot apply as much force as a solid wrench.
It should be mentioned, if mostly to condemn the practice, that pliers are sometimes used to adjust nuts. Most types of pliers, however, do not have parallel jaws, but angled ones. These are apt to deform a nut if heavy pressure is applied.
Pipe wrench
Pipe wrenches grasp threaded pipes and turn them, so they do not have flat jaws. One common type has curved jaws, with the wrench designed to slip slightly so it can be repositioned as the pipe moves under it. These were called Stillson wrenches after the inventor; they are sometimes, incorrectly, called monkey wrenches.
Another type does not have jaws, but instead a chain that tensions against the pipe.
Some plumbing fixtures have a nut-like shape molded into the metal, so that a flat-jawed wrench can be used. This is the application intended for the increasingly rare monkey wrench.
Socket wrenches and nutdrivers
Sockets proper are single pieces of cast or machined metal, with one end shaped to slip over the fastener to be manipulated, and the other to receive a driveshaft to turn it. The shaft end may actually have a depression, or even a spring-loaded bearing, to help lock the shaft into place. In combination, the driveshaft and socket are extremely strong in rotation, but should separate easily with linear traction.
Where the English measurement system is used, the shaft diameter is most often 3/8" or 1/4" for general purposes. 1/2", 3/4", and 1" drives are used for heavy equipment. Both metric and English system sockets, however, can snap onto the male end of the shaft.
Adapters are available to convert between shaft sizes. For example, one might have a large nut but only a 1/4" shaft system, so an adapter can allow a 3/8" socket to be used with that shaft.
Basic handle and extension
The most basic driver is a shaft with a handle, much like a screwdriver, but with a socket-mating connector rather than a screwdriver bit. Another very common variant, called an extension, has a male connector that mates with the socket at one end, and a female connector that accepts another socket tool at the other. Multiple extensions can be snapped together for extra length.
Nutdrivers
Nutdrivers are a permanently attached set of socket, driveshaft, and handle. Some nutdrivers have hollow shafts, so bolt length protruding above the nut goes into the hollow and does not interfere with rotation.
Aids to leverage
Most often, however, at the end of the extension away from the socket, a tool to improve the leverage of driving is attached. One such tool is a ratchet, which, at first, looks like an open-ended or box wrench. The male connector, however, is attached to a disk and mechanical components inside the ratchet head. There is a small control that selects the direction (i.e., loosening or tightening) in which the socket is to be moved. Once that control is set, the ratchet moves freely in the other direction, so it can be repositioned easily; it is not necessary to have 360 degree access above the fastener -- just enough working space to move the ratchet handle.
Other ways to improve leverage include various pivoting handles. One type looks like the brace (tool) used to drive hand drills; there is a socket fitting rather than a chuck at the working end. The operator holds a knob, fitted with bearings in which the shaft turns, and cranks a handle.
Another type, called a flex handle, is related to the rachet, but, rather than rotating, the handle pivots 180 degrees so it can be repositioned quickly. A flex handle is simpler, cheaper, and may be able to apply more force than a rachet.
Torque wrenches
A torque wrench is both a fastening tool and a measuring device. It is used where precise measurement of the tightness of a bolt is necessary.
It has been suggested that a quantum mechanical torque wrench can measure either the torque, or find the bolt, but not both.
Spanners (precision)
While a spanner is a general term in British English for "wrench", there is a specialized tool always known as a spanner. It consists of a bar with two or more protruding pins, sometimes adjustable in distance, that fit into corresponding holes in a fastener an allow it to be turned. The bar is connected to a shaft or other mechanism to allow turning, or sometimes the spanner is built not as a bar, but as a pliers-like device with the pins at the work end. Spanners are often used in optical work, on the retaining rings of lens elements.
Power wrenches
Impact wrenches
One of the most common types is a power-operated driver for sockets, the sockets usually made of extra-strong metal. Pneumatically driven impact wrenches are extremely common in the automotive industry; air drive does not generate the sparks that an electric motor could produce, which would be hazardous in the presence of petroleum products.
For other applications, however, electric impact wrenches are increasingly common, especially cordless battery-powered wrenches with great portability. For some heavy applications, the impact energy may be provided by an explosive cartridge.
There are impact wrenches that are hand-powered, but by hitting a specific part with a hammer, allowing great force to be applied.
Power torque wrench
Intended for industrial applications, these are often powered hydraulically, which allows great precision. (Read more...)
Previous Winners
 Air preheater: A general term to describe any device designed to preheat the combustion air used in a fuel-burning furnace for the purpose of increasing the thermal efficiency of the furnace. [e] (June 11) Air preheater: A general term to describe any device designed to preheat the combustion air used in a fuel-burning furnace for the purpose of increasing the thermal efficiency of the furnace. [e] (June 11) 2009 H1N1 influenza virus: A contagious influenza A virus discovered in April 2009, commonly known as swine flu. [e] (June 4) 2009 H1N1 influenza virus: A contagious influenza A virus discovered in April 2009, commonly known as swine flu. [e] (June 4) Gasoline: A fuel for spark-ignited internal combustion engines derived from petroleum crude oil. [e] (21 May) Gasoline: A fuel for spark-ignited internal combustion engines derived from petroleum crude oil. [e] (21 May) John Brock: Fictional British secret agent who starred in three 1960s thrillers by Desmond Skirrow. [e] (8 May) John Brock: Fictional British secret agent who starred in three 1960s thrillers by Desmond Skirrow. [e] (8 May) McGuffey Readers: A set of highly influential school textbooks used in the 19th and early 20th centuries in the elementary grades in the United States. [e] (14 Apr) McGuffey Readers: A set of highly influential school textbooks used in the 19th and early 20th centuries in the elementary grades in the United States. [e] (14 Apr) Vector rotation: Process of rotating one unit vector into a second unit vector. [e] (7 Apr) Vector rotation: Process of rotating one unit vector into a second unit vector. [e] (7 Apr) Leptin: Hormone secreted by adipocytes that regulates appetite. [e] (31 Mar) Leptin: Hormone secreted by adipocytes that regulates appetite. [e] (31 Mar) Kansas v. Crane: A 2002 decision of the Supreme Court of the United States, ruling that a person could not be adjudicated a sexual predator and put in indefinite medical confinement, purely on assessment of an emotional disorder, but such action required proof of a likelihood of uncontrollable impulse presenting a clear and present danger. [e] (24 Mar) Kansas v. Crane: A 2002 decision of the Supreme Court of the United States, ruling that a person could not be adjudicated a sexual predator and put in indefinite medical confinement, purely on assessment of an emotional disorder, but such action required proof of a likelihood of uncontrollable impulse presenting a clear and present danger. [e] (24 Mar) Punch card: A term for cards used for storing information. Herman Hollerith is credited with the invention of the media for storing information from the United States Census of 1890. [e] (17 Mar) Punch card: A term for cards used for storing information. Herman Hollerith is credited with the invention of the media for storing information from the United States Census of 1890. [e] (17 Mar) Jass–Belote card games: A group of trick-taking card games in which the Jack and Nine of trumps are the highest trumps. [e] (10 Mar) Jass–Belote card games: A group of trick-taking card games in which the Jack and Nine of trumps are the highest trumps. [e] (10 Mar) Leptotes (orchid): A genus of orchids formed by nine small species that exist primarily in the dry jungles of South and Southeast Brazil. [e] (3 Mar) Leptotes (orchid): A genus of orchids formed by nine small species that exist primarily in the dry jungles of South and Southeast Brazil. [e] (3 Mar) Worm (computers): A form of malware that can spread, among networked computers, without human interaction. [e] (24 Feb) Worm (computers): A form of malware that can spread, among networked computers, without human interaction. [e] (24 Feb) Joseph Black: (1728 – 1799) Scottish physicist and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide [e] (11 Feb 2009) Joseph Black: (1728 – 1799) Scottish physicist and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide [e] (11 Feb 2009) Sympathetic magic: The cultural concept that a symbol, or small aspect, of a more powerful entity can, as desired by the user, invoke or compel that entity [e] (17 Jan 2009) Sympathetic magic: The cultural concept that a symbol, or small aspect, of a more powerful entity can, as desired by the user, invoke or compel that entity [e] (17 Jan 2009) Dien Bien Phu: Site in northern Vietnam of a 1954 decisive battle that soon forced France to relinquish control of colonial Indochina. [e] (25 Dec) Dien Bien Phu: Site in northern Vietnam of a 1954 decisive battle that soon forced France to relinquish control of colonial Indochina. [e] (25 Dec) Blade Runner: 1982 science fiction film directed by Ridley Scott and starring Harrison Ford, set in an imagined Los Angeles of 2019. [e] (25 Nov) Blade Runner: 1982 science fiction film directed by Ridley Scott and starring Harrison Ford, set in an imagined Los Angeles of 2019. [e] (25 Nov) Piquet: A two-handed card game played with 32 cards that originated in France around 1500. [e] (18 Nov) Piquet: A two-handed card game played with 32 cards that originated in France around 1500. [e] (18 Nov) Crash of 2008: the international banking crisis that followed the subprime mortgage crisis of 2007. [e] (23 Oct) Crash of 2008: the international banking crisis that followed the subprime mortgage crisis of 2007. [e] (23 Oct)- Information Management: Add brief definition or description (31 Aug)
 Battle of Gettysburg: A turning point in the American Civil War, July 1-3, 1863, on the outskirts of Gettysburg, Pennsylvania. [e] (8 July) Battle of Gettysburg: A turning point in the American Civil War, July 1-3, 1863, on the outskirts of Gettysburg, Pennsylvania. [e] (8 July) Drugs banned from the Olympics: Substances prohibited for use by athletes prior to, and during competing in the Olympics. [e] (1 July) Drugs banned from the Olympics: Substances prohibited for use by athletes prior to, and during competing in the Olympics. [e] (1 July) Sea glass: Formed when broken pieces of glass from bottles, tableware, and other items that have been lost or discarded are worn down and rounded by tumbling in the waves along the shores of oceans and large lakes. [e] (24 June) Sea glass: Formed when broken pieces of glass from bottles, tableware, and other items that have been lost or discarded are worn down and rounded by tumbling in the waves along the shores of oceans and large lakes. [e] (24 June) Dazed and Confused (Led Zeppelin song): Landmark 1969 song recorded by Led Zeppelin for their eponymous debut album, which became an early centrepiece for the group's live performances. [e] (17 June) Dazed and Confused (Led Zeppelin song): Landmark 1969 song recorded by Led Zeppelin for their eponymous debut album, which became an early centrepiece for the group's live performances. [e] (17 June) Hirohito: The 124th and longest-reigning Emperor of Japan, 1926-89. [e] (10 June) Hirohito: The 124th and longest-reigning Emperor of Japan, 1926-89. [e] (10 June) Henry Kissinger: (1923—) American academic, diplomat, and simultaneously Assistant to the President for National Security Affairs and Secretary of State in the Nixon Administration; promoted realism (foreign policy) and détente with China and the Soviet Union; shared 1973 Nobel Peace Prize for ending the Vietnam War; Director, Atlantic Council [e] (3 June) Henry Kissinger: (1923—) American academic, diplomat, and simultaneously Assistant to the President for National Security Affairs and Secretary of State in the Nixon Administration; promoted realism (foreign policy) and détente with China and the Soviet Union; shared 1973 Nobel Peace Prize for ending the Vietnam War; Director, Atlantic Council [e] (3 June) Palatalization: An umbrella term for several processes of assimilation in phonetics and phonology, by which the articulation of a consonant is changed under the influence of a preceding or following front vowel or a palatal or palatalized consonant. [e] (27 May) Palatalization: An umbrella term for several processes of assimilation in phonetics and phonology, by which the articulation of a consonant is changed under the influence of a preceding or following front vowel or a palatal or palatalized consonant. [e] (27 May) Intelligence on the Korean War: The collection and analysis, primarily by the United States with South Korean help, of information that predicted the 1950 invasion of South Korea, and the plans and capabilities of the enemy once the war had started [e] (20 May) Intelligence on the Korean War: The collection and analysis, primarily by the United States with South Korean help, of information that predicted the 1950 invasion of South Korea, and the plans and capabilities of the enemy once the war had started [e] (20 May) Trinity United Church of Christ, Chicago: A predominantly black church located in south Chicago with upwards of 10,000 members, established in 1961. [e] (13 May) Trinity United Church of Christ, Chicago: A predominantly black church located in south Chicago with upwards of 10,000 members, established in 1961. [e] (13 May) BIOS: Part of many modern computers responsible for basic functions such as controlling the keyboard or booting up an operating system. [e] (6 May) BIOS: Part of many modern computers responsible for basic functions such as controlling the keyboard or booting up an operating system. [e] (6 May) Miniature Fox Terrier: A small Australian vermin-routing terrier, developed from 19th Century Fox Terriers and Fox Terrier types. [e] (23 April) Miniature Fox Terrier: A small Australian vermin-routing terrier, developed from 19th Century Fox Terriers and Fox Terrier types. [e] (23 April) Joseph II: (1741–1790), Holy Roman Emperor and ruler of the Hapsburg (Austrian) territories who was the arch-embodiment of the Enlightenment spirit of the later 18th-century reforming monarchs. [e] (15 Apr) Joseph II: (1741–1790), Holy Roman Emperor and ruler of the Hapsburg (Austrian) territories who was the arch-embodiment of the Enlightenment spirit of the later 18th-century reforming monarchs. [e] (15 Apr) British and American English: A comparison between these two language variants in terms of vocabulary, spelling and pronunciation. [e] (7 Apr) British and American English: A comparison between these two language variants in terms of vocabulary, spelling and pronunciation. [e] (7 Apr) Count Rumford: Add brief definition or description (1 April) Count Rumford: Add brief definition or description (1 April) Whale meat: Add brief definition or description (25 March) Whale meat: Add brief definition or description (25 March) Naval guns: Add brief definition or description (18 March) Naval guns: Add brief definition or description (18 March) Sri Lanka: Add brief definition or description (11 March) Sri Lanka: Add brief definition or description (11 March) Led Zeppelin: Add brief definition or description (4 March) Led Zeppelin: Add brief definition or description (4 March) Martin Luther: Add brief definition or description (20 February) Martin Luther: Add brief definition or description (20 February) Cosmology: Add brief definition or description (4 February) Cosmology: Add brief definition or description (4 February) Ernest Rutherford: Add brief definition or description(28 January) Ernest Rutherford: Add brief definition or description(28 January) Edinburgh: Add brief definition or description (21 January) Edinburgh: Add brief definition or description (21 January) Russian Revolution of 1905: Add brief definition or description (8 January 2008) Russian Revolution of 1905: Add brief definition or description (8 January 2008) Phosphorus: Add brief definition or description (31 December) Phosphorus: Add brief definition or description (31 December) John Tyler: Add brief definition or description (6 December) John Tyler: Add brief definition or description (6 December) Banana: Add brief definition or description (22 November) Banana: Add brief definition or description (22 November) Augustin-Louis Cauchy: Add brief definition or description (15 November) Augustin-Louis Cauchy: Add brief definition or description (15 November)- B-17: Add brief definition or description - 8 November 2007
 Red Sea Urchin: Add brief definition or description - 1 November 2007 Red Sea Urchin: Add brief definition or description - 1 November 2007 Symphony: Add brief definition or description - 25 October 2007 Symphony: Add brief definition or description - 25 October 2007 Oxygen: Add brief definition or description - 18 October 2007 Oxygen: Add brief definition or description - 18 October 2007 Origins and architecture of the Taj Mahal: Add brief definition or description - 11 October 2007 Origins and architecture of the Taj Mahal: Add brief definition or description - 11 October 2007 Fossilization (palaeontology): Add brief definition or description - 4 October 2007 Fossilization (palaeontology): Add brief definition or description - 4 October 2007 Cradle of Humankind: Add brief definition or description - 27 September 2007 Cradle of Humankind: Add brief definition or description - 27 September 2007 John Adams: Add brief definition or description - 20 September 2007 John Adams: Add brief definition or description - 20 September 2007 Quakers: Add brief definition or description - 13 September 2007 Quakers: Add brief definition or description - 13 September 2007 Scarborough Castle: Add brief definition or description - 6 September 2007 Scarborough Castle: Add brief definition or description - 6 September 2007 Jane Addams: Add brief definition or description - 30 August 2007 Jane Addams: Add brief definition or description - 30 August 2007 Epidemiology: Add brief definition or description - 23 August 2007 Epidemiology: Add brief definition or description - 23 August 2007 Gay community: Add brief definition or description - 16 August 2007 Gay community: Add brief definition or description - 16 August 2007 Edward I: Add brief definition or description - 9 August 2007 Edward I: Add brief definition or description - 9 August 2007
Rules and Procedure
Rules
- The primary criterion of eligibility for a new draft is that it must have been ranked as a status 1 or 2 (developed or developing), as documented in the History of the article's Metadate template, no more than one month before the date of the next selection (currently every Thursday).
- Any Citizen may nominate a draft.
- No Citizen may have nominated more than one article listed under "current nominees" at a time.
- The article's nominator is indicated simply by the first name in the list of votes (see below).
- At least for now--while the project is still small--you may nominate and vote for drafts of which you are a main author.
- An article can be the New Draft of the Week only once. Nominated articles that have won this honor should be removed from the list and added to the list of previous winners.
- Comments on nominations should be made on the article's talk page.
- Any draft will be deleted when it is past its "last date eligible". Don't worry if this happens to your article; consider nominating it as the Article of the Week.
- If an editor believes that a nominee in his or her area of expertise is ineligible (perhaps due to obvious and embarrassing problems) he or she may remove the draft from consideration. The editor must indicate the reasons why he has done so on the nominated article's talk page.
Nomination
See above section "Add New Nominees Here".
Voting
- To vote, add your name and date in the Supporters column next to an article title, after other supporters for that article, by signing
<br />~~~~. (The date is necessary so that we can determine when the last vote was added.)
- Add your name in the Specialist supporters column only if you are an editor who is an expert about the topic in question. Your vote will be counted as three.
- You may vote for as many articles as you wish, and each vote counts separately, but you can only nominate one at a time; see above. You could, theoretically, vote for every nominated article on the page, but this would be pointless.
Ranking
- The list of articles is sorted by number of votes first, then alphabetically.
- Admins should make sure that the votes are correctly tallied, but anyone may do this. Note that "Specialist Votes" are worth 3 points.
Updating
- Each Thursday, one of the admins listed below should move the winning article to the Current Winner section of this page, announce the winner on Citizendium-L and update the "previous winning drafts" section accordingly.
- The winning article will be the article at the top of the list (ie the one with the most votes).
- In the event of two or more having the same number of votes :
- The article with the most specialist supporters is used. Should this fail to produce a winner, the article appearing first by English alphabetical order is used.
- The remaining winning articles are guaranteed this position in the following weeks, again in alphabetical order. No further voting should take place on these, which remain at the top of the table with notices to that effect. Further nominations and voting take place to determine future winning articles for the following weeks.
- Winning articles may be named New Draft of the Week beyond their last eligible date if their circumstances are so described above.
Administrators
The Administrators of this program are the same as the admins for CZ:Article of the Week.
References
See Also
|
|


















