User talk:Nicholas Siebenlist
Welcome!
Welcome to the Citizendium! We hope you will contribute boldly and well. Here are pointers for a quick start. You'll probably want to know how to get started as an author. Just look at CZ:Getting Started for other helpful "startup" links, and CZ:Home for the top menu of community pages. Be sure to stay abreast of events via the Citizendium-L (broadcast) mailing list (do join!) and the blog. Please also join the workgroup mailing list(s) that concern your particular interests. You can test out editing in the sandbox if you'd like. If you need help to get going, the forums is one option. That's also where we discuss policy and proposals. You can ask any constable for help, too. Me, for instance! Just put a note on their "talk" page. Again, welcome and have fun! D. Matt Innis 01:11, 9 November 2010 (UTC)
Posting your article
Hi Nick, it looks as if you have composed your article entirely here on your talk page. Would you kindly transfer it to the main wiki page for Adsorption so that it can be seen and read. I would do it myself but if I do it, it will show up as my contribution when it is really yours. Thanks! Jean B. Hunter 09:36, 22 December 2010 (UTC)
Links to Wiki Articles
http://en.wikipedia.org/wiki/HPLC http://en.wikipedia.org/wiki/Differential_centrifugation http://en.wikipedia.org/wiki/Liquid-liquid_extraction http://en.wikipedia.org/wiki/Partition_coefficient http://en.wikipedia.org/wiki/Sieving_coefficient http://en.wikipedia.org/wiki/Filtration http://en.wikipedia.org/wiki/Cross_flow_filtration http://en.wikipedia.org/wiki/Protein_purification http://en.wikipedia.org/wiki/Size_Exclusion_Chromatography http://en.wikipedia.org/wiki/Immunoprecipitation
Adsorption
Adsorption
Adsorption is the binding of target molecules to a surface. The target molecules can be bound based on different chemical or physical properties. A wide variety of separation techniques are therefore available and various molecules that can be targeted (ions, gas, DNA, etc.). Binding of target molecules occurs with differential strengths based on the type of interactions and the specificity of the interaction. The binding is reversible and the unbinding between molecule and adsorbent is known as desorption.
Adsorption is used in many chemical and biological processes, natural and synthetic. Chromatography is based on the principle of adsorption.
Isotherms
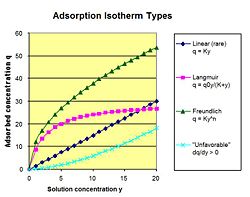
An isotherm is the relationship between the adsorbed concentration of a molecule in solution and the non-adsorbed concentration of the molecule. The relationship is determined by pressure, temperature, and mass of the adsorbent. The slope of an Isotherm at a particular concentration is the partition coefficient K. The ratio of partition coefficients between two molecules is the selectivity ratio β.
(Dr. Hunter)
Linear The linear isotherm is the simplest of the isotherms. The relationship between the absorbed concentration q and the liquid or gas concentration y is q = Ky. K is empirically determined based on the strength of interaction between the molecule and the adsorbent. A molecule that does not bind would have a K value of 0 at every temperature, pressure, etc.
Langmuir The Langmuir Isotherm has a maximum number of accessible sites for a molecule to bind. An equilibrium is established between the molecules in solution and those in filled sites. The relationship is q = q0y / (K + y) (I plan on changing this with equation editor). Q0 and K are empirically determined.
Given the absorbed concentration q at various liquid concentrations y, the empirical constants K and n can be determined. Plotting 1/q versus 1/ y yields an intercept equal to 1/q0 and a slope of K/q0.
Freundlich The Freundlich Isotherm has the relationship: q = Ky^n
Given the absorbed concentration q at various liquid concentrations y, the empirical constants K and n can be determined. Plotting the natural log of q versus the natural log of y yields an intercept equal to the natural log of K and a slope of n.
Column
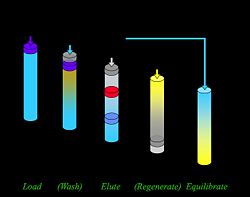
Column adsorption is used in the separation of target molecules from impurities. Different affinity types such as ion exchange and hydrophobic are available and each yields different separation properties. Certain general characteristics regarding separation and solvent movement apply to all types.
(Dr. Hunter)
Equipment Each column contains a stationary phase onto which material is adsorbed and a mobile phase which flows through the column, carrying targets and impurities. The stationary phase is bound to the column in such a way that it does not move during elution, although moving solutes do bind and then release from it. The mobile phase is chosen to limit interactions with the stationary phase so the eluent flows through unhindered. When choosing the mobile phase, solubility and viscosity must also be considered.
Retention Time The retention time is the time between the application of target solution and the peak elution of a molecule. The retention time of the solvent (eluent) is known as t0. When there is no molecular interaction between the solvent and the stationary phase, the solvent retention time can be calculated knowing the column volume, the flow rate, and the void space (packing density related). t0 = (V∈)/Q V = volume, ε = void fraction, Q = flow rate.
Distribution of a molecule during elution is simplified by the plate model. This model assumes that a column can be divided into many identical stacked plates. When the mass balance is performed on each plate during elution, the resulting distribution of a molecule during elution is a Gaussian like curve. By approximating the curve as such more information about the elution is attained. The curve width is estimated as 4 times the standard deviation of the curve.
Resolution The resolution between two molecules during an elution provides a quantitative measurement of how good the separation between the two is. Resolution is calculated knowing the retention time of each molecule and the width of each peak. Rs = (2(rentention time A-retention time B))/((width peak A+width peak B)) The theoretical plates of a column can also be calculated from the same parameters.
N (number of theoretical plates) = ((retention time of molecule)/(standard deviation of peak))2
A larger calculated resolution is equivalent to a better separation (higher purity). The larger the number of theoretical plates, the better a separation will be and the narrower a peak will be.
Expanded Bed
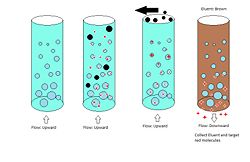
Expanded bed adsorption works on the same principles as column chromatography but uses reverse flow and differently packed materials. Expanded bed is used typically when column chromatography is not effective or possible because of a very high binding constant K, when particulates are present, or when a very high flow rate is needed. Advantages to this procedure over column adsorption are the elimination of a filtration step and improved yield. A disadvantage to this procedure is more complicated equipment, particularly a control center which must reverse the flow.
Procedure Expanded bed adsorption is a multistep procedure. The column is filled with packing as in column chromatography but in this case the void space is much higher (~0.8 instead of 0.3) and the packing is of variable sizes instead of a single size. The first step is a driving of the target solution over the bed in an upwards direction. The high void volume and mixed bead sizes allow for the application of slurries, removing a previously necessary filtration step. Particulate matter, e.g. Cells and debris flow through the bed and out when they would have previously blocked a packed column. The target molecules adhere to the beads and are retained during the first step. The second step may be a wash which does not remove the target but helps to remove remaining particles not intended for separation. The final step is a typical elution. The flow is reversed to the conventional downward direction and an eluent is used to remove the target molecules from the beads. Retention times and peak widths are not applicable here because of the starting elution distribution. The target will already be purified in this process.
Batch Absorption
Batch absorption is similar to column chromatography in the principles of separating based on differences in affinity but a column is not used. Advantages to this method over column absorption are no need for a column and greater purity due to an unlimited number of washing steps if chosen. Disadvantages are a need for a centrifuge and decreased yield from washing.
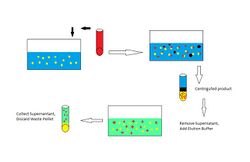
Procedure To start, mix the target solution with a solid phase which will selectivity bind the target molecules and not the impurities. The depleted feed is then removed, typically following a centrifugation or filtration to collect the solid particles. A wash buffer is added and again centrifuged to remove impurities. A third elution buffer is then added which will selectively remove the target molecules. A third centrifugation is done and the supernatant containing the target is retained.
Biological example Cells containing a target protein are lysed and mixed with beads which have an antibody for the protein attached to the surface. The solution is filtered to remove particulate matter. The mixture is centrifuged and the heavy beads are retained while supernatant is removed. A washing buffer is added and centrifuged to remove remaining impurities. An elution buffer is added and centrifuged. The supernatant contains the target protein.
References Mattiasson, B. & Nandakumar, M. P. Physicochemical Basis of Expanded-Bed Adsorption for Protein Purification. Handbook of Bioseparations, Vol. 2: Separation Science and Technology; S. Ahuja, Ed.; Academic Press: San Diego, 2000. "column chromatography." Encyclopædia Britannica. 2010. Encyclopædia Britannica Online. 16 Dec. 2010 <http://www.britannica.com/EBchecked/topic/127156/column-chromatography>. Rutherford, S.W. & Do, D.D. (2000) “Adsorption dynamics measured by permeation and batch adsorption” Chemical Engineering Journal. 76 (1), p23-31.