Diabesity: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
The term '''diabesity''' was coined by Ethan Sims in 1973, to describe the close relationship between [[type 2 diabetes]] (T2DM) and [[obesity]]. Their findings suggested that by overfeeding young men, with no previous family history of diabetes, the initial signs of diabetes were induced. This excess consuption led to increases in insulin production, plasma glucose, triglycerides and eventually impaired glucose tolerance; all signs predisposing one to T2DM and obesity<ref> | |||
Sims EAH ''et al.'' (1973) Endocrine and metabolic effects of experimental obesity in man, ''Recent Prog Horm Res'' 29:457–96</ref><ref>Haslam DW, James WP (2005) Obesity''Lancet'' 366:1197–209</ref> | |||
[[Diabetes mellitus type 2]] is a disorder where cells fail to take up [[glucose]] from the blood. Glucose is the fuel for respiration which produces energy for our cells to function properly. As there is no glucose entering the cells, they waste away and are forced to consume their own proteins. Diabetes is the foremost cause of kidney failure ([[diabetic nephropathy]]), blindness ([[diabetic retinopathy]]), and amputation in adults ([[diabetic neuropathy]]). People with this disease lack the ability to utilize the hormone [[insulin]]. Insulin is produced by the [[pancreas]] after a meal inresponse to increased concentrations of glucose in the blood. The insulin signal attaches to specific receptors on the surface of target cells, causing them to switch on their glucose-transporting machinery. Type 2 diabetics have normal or even elevated levels of insulin in their blood, and normal insulin receptors, but the binding of insulin to its receptors does not turn on the glucose-transporting machinery. | |||
[[Diabetes mellitus type 2]] is a disorder where cells fail to take up [[glucose]] from the blood. Glucose is the fuel for respiration which produces energy for our cells to function properly. As there is no glucose entering the cells, they waste away and are forced to consume their own proteins. Diabetes is the foremost cause of kidney failure ([[diabetic nephropathy]]), blindness ([[diabetic retinopathy]]), and amputation in adults ([[diabetic neuropathy]]). | |||
People with this disease lack the ability to utilize the hormone [[insulin]]. Insulin is produced by the [[pancreas]] after a meal inresponse to increased concentrations of glucose in the blood. The insulin signal attaches to specific receptors on the surface of target cells, causing them to switch on their glucose-transporting machinery. Type 2 diabetics have normal or even elevated levels of insulin in their blood, and normal insulin receptors, but the binding of insulin to its receptors does not turn on the glucose-transporting machinery. | |||
Proteins called [[IRS proteins]] (insulin receptor substrate) bind with the insulin receptor inside the cell. The receptor responds by adding a phosphate group onto the IRS molecules. This rouses the IRS molecules into action, and they activate a variety of processes, including an enzyme that turns on the glucose transporter machinery. When the IRS genes are deliberately inactivated in [[transgenic]] “knockout” mice, type 2 diabetes results. However, there are no IRS gene mutations in inherited type 2 diabetics; the IRS genes are normal. This suggests that in type 2 diabetes something is impeding with the action of the IRS proteins. An estimated 80% of those who develop type 2 diabetes are obese, an enticing association. | Proteins called [[IRS proteins]] (insulin receptor substrate) bind with the insulin receptor inside the cell. The receptor responds by adding a phosphate group onto the IRS molecules. This rouses the IRS molecules into action, and they activate a variety of processes, including an enzyme that turns on the glucose transporter machinery. When the IRS genes are deliberately inactivated in [[transgenic]] “knockout” mice, type 2 diabetes results. However, there are no IRS gene mutations in inherited type 2 diabetics; the IRS genes are normal. This suggests that in type 2 diabetes something is impeding with the action of the IRS proteins. An estimated 80% of those who develop type 2 diabetes are obese, an enticing association. | ||
==The Genetics of Diabesity== | ==The Genetics of Diabesity== | ||
===Thrifty gene hypothesis === | ===Thrifty gene hypothesis === | ||
The [[thrifty gene hypothesis]] | The [[thrifty gene hypothesis]] remains a popular explanation for the growing rise of diabetes and obesity. | ||
In 1962, <ref>The original | In 1962, <ref>The original paper that proposes the thrifty genotype. | ||
Neel JV (1962) Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"?. ''Am J Hum Genet'' 14:353–62</ref> Neel proposed this hypothesis to explain the high prevalence of T2D in recently westernized nations. He suggested that the “thrifty” genes that predispose to T2D and obesity are evolutionary advantageous. He proposed that in the primitive age, where famine and food shortages would be common, individuals with “thrifty genes” were more likely to survive. This is because these individuals could store a high percentage of their energy as fat, and use this energy store to survive times of famine. However, in the modern world there is a constant abundance of food; the thrifty genes prepare us for a famine that never comes, resulting in a huge rise in T2D and obesity <ref>Joffe B, Zimmet P (1998) | Neel JV (1962) Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"?. ''Am J Hum Genet'' 14:353–62</ref> Neel proposed this hypothesis to explain the high prevalence of T2D in recently westernized nations. He suggested that the “thrifty” genes that predispose to T2D and obesity are evolutionary advantageous. He proposed that in the primitive age, where famine and food shortages would be common, individuals with “thrifty genes” were more likely to survive. This is because these individuals could store a high percentage of their energy as fat, and use this energy store to survive times of famine. However, in the modern world there is a constant abundance of food; the thrifty genes prepare us for a famine that never comes, resulting in a huge rise in T2D and obesity <ref>Joffe B, Zimmet P (1998) The thrifty genotype in type 2 diabetes. ''Endocrine'' 9:139–41</ref> | ||
Those from ethnic populations who had experienced famine and poor nutrition until relatively recently, are shown to have the '''highest rates''' in the world (3) | |||
<ref>Relates genetic and environmental factors. Thrifty gene hypothesis summarised | <ref>Relates genetic and environmental factors. Thrifty gene hypothesis summarised | ||
Noel Cameron, Nicholos G Norgan, George Ellison, Childhood Obesity Contemporary Issues 2006, CRC press, Taylor and Francis Group. </ref>For example, research on the [[Pima Indians]] has contributed in providing clear evidence that a sudden abundance in food can lead to a substantial rise in obesity rates as well as T2D rates. The research found that 50% of adult Pima Indians have diabetes, and 95% of these were overweight. <ref>Website which describes the study on Pima Indians - rates of Obesity and Diabetes http://diabetes.niddk.nih.gov/DM/pubs/pima/obesity/obesity.htm</ref> | Noel Cameron, Nicholos G Norgan, George Ellison, Childhood Obesity Contemporary Issues 2006, CRC press, Taylor and Francis Group. </ref>For example, research on the [[Pima Indians]] has contributed in providing clear evidence that a sudden abundance in food can lead to a substantial rise in obesity rates as well as T2D rates. The research found that 50% of adult Pima Indians have diabetes, and 95% of these were overweight. <ref>Website which describes the study on Pima Indians - rates of Obesity and Diabetes http://diabetes.niddk.nih.gov/DM/pubs/pima/obesity/obesity.htm</ref> | ||
==Visceral fat accumulation and type 2 diabetes== | ==Visceral fat accumulation and type 2 diabetes== | ||
Excess visceral adipose tissue increases the risk for | Excess visceral adipose tissue increases the risk for type 2 diabetes. Recently it has been discovered that excess fat within the abdomen, known as visceral adiposity, creates a serious independent health risk of metabolic complications than accumulation of adipose tissue in other regions. Visceral adiposity is related with an increase in ''insulin resistance'', whereas abdominal subcutaneous fat is not. (''Insulin resistance'' describes the impaired ability of insulin to suppress hepatic glucose output and promote glucose disposal in the periphery.) As diabetes gets worse, patients have higher blood sugar levels (hyperglycaemia) because their beta cells, in the pancreas, are unable to keep making enough insulin. In insulin resistance, normal amounts of insulin are unable to produce a normal response from adipose, muscle and liver cells. | ||
As diabetes gets worse, patients have higher blood sugar levels (hyperglycaemia) because their beta cells, in the pancreas, are unable to keep making enough insulin. In insulin resistance, normal amounts of insulin are unable to produce a normal response from adipose, muscle and liver cells. | |||
In 1994, a new hormone was found, called [[leptin]], that | In 1994, a new hormone was found, called [[leptin]], that provides feedback to the brain of the level of fat in the body. Leptin suppresses appetite, but most obese people have very high leptin levels, as leptin is secreted by adipose cells. Therefore, obesity is not generally caused by a deficiency in leptin; instead there seems to be a defect in leptin signalling. | ||
Cnop et al showed that visceral fat is the best predictor of insulin sensitivity whilst subcutaneous fat establishes leptin levels [9]. | Cnop et al showed that visceral fat is the best predictor of insulin sensitivity whilst subcutaneous fat establishes leptin levels [9]. | ||
Adipocytes produce an array of other peptides including [[adiponectin]], [[resistin]] and [[TNF alpha]] 25. They are act on peripheral tissue and thereby affect insulin sensitivity and the processes involved in substrate metabolism. As discussed later, resistin could be one of the key connections between obesity and DM2 by inhibiting adipogenesis (formation of adipse tissue) and increasing the circulating [[free fatty acids]]. | Adipocytes produce an array of other peptides including [[adiponectin]], [[resistin]] and [[TNF alpha]] 25. They are act on peripheral tissue and thereby affect insulin sensitivity and the processes involved in substrate metabolism. As discussed later, resistin could be one of the key connections between obesity and DM2 by inhibiting adipogenesis (formation of adipse tissue) and increasing the circulating [[free fatty acids]]. Adipose tissue is an important organ that can regulate energy balance through the complex control of hormone and neuronal signals using leptin, adiponectin, resistin, TNF alpha, and angiotensin. | ||
Adipose tissue is an important organ that can regulate energy balance through the complex control of hormone and neuronal signals using leptin, adiponectin, resistin, TNF alpha, and angiotensin. | |||
The lipoprotein profile related to obesity and insulin resistance is mostly due to intra-abdominal fat [4]. There are better measures of obesity (particularly visceral obesity) that can predict diabetes. These include ''waist circumference'', the ''waist-to-hip ratio'' and ''insulin resistance''. | The lipoprotein profile related to obesity and insulin resistance is mostly due to intra-abdominal fat [4]. There are better measures of obesity (particularly visceral obesity) that can predict diabetes. These include ''waist circumference'', the ''waist-to-hip ratio'' and ''insulin resistance''. | ||
| Line 42: | Line 32: | ||
Intramyocellular lipids (IMCL) are more closely associated to insulin resistance than to body mass index, waist-to-hip ratio, or total body fat. High free fatty acid and VLDL levels are a key cause of fat accumulation in muscle cells and IMCL increases have been seen in patients with insulin resistance. | Intramyocellular lipids (IMCL) are more closely associated to insulin resistance than to body mass index, waist-to-hip ratio, or total body fat. High free fatty acid and VLDL levels are a key cause of fat accumulation in muscle cells and IMCL increases have been seen in patients with insulin resistance. | ||
Surgical removal of visceral fat had a positive effect on the hepatic and peripheral insulin sensitivity, and on leptin and TNFα levels. [6] | Surgical removal of visceral fat had a positive effect on the hepatic and peripheral insulin sensitivity, and on leptin and TNFα levels. [6] | ||
| Line 50: | Line 39: | ||
[[11β-Hydroxysteroid dehydrogenase type 1]] (11HSD-1) catalyses the conversion of inactive [[cortisone]] to active [[cortisol]], a potent [[glucocorticoid]]. It is found throughout the body and is a highly regulated enzyme that increases the ligand accessibility for glucocorticoid receptors. | [[11β-Hydroxysteroid dehydrogenase type 1]] (11HSD-1) catalyses the conversion of inactive [[cortisone]] to active [[cortisol]], a potent [[glucocorticoid]]. It is found throughout the body and is a highly regulated enzyme that increases the ligand accessibility for glucocorticoid receptors. | ||
{{Image|Diabesity.jpg|right|500px|}} | {{Image|Diabesity.jpg|right|500px|}} | ||
Excessive glucocorticoid exposure causes central obesity, [[hypertension]], and [[dyslipidaemia]] and insulin resistance, as seen in [[Cushing’s syndrome]]. Transgenic mice over-expressing 11HSD1 in | Excessive glucocorticoid exposure causes central obesity, [[hypertension]], and [[dyslipidaemia]] and insulin resistance, as seen in [[Cushing’s syndrome]]. Transgenic mice over-expressing 11HSD1 in white adipose tissue have these features as well, while 11HSD1 knockout mice are protected from these metabolic abnormalities. In human idiopathic obesity, circulating cortisol levels are not elevated but 11HSD1 mRNA and activity is increased in subcutaneous adipose. The impact of increased adipose 11HSD1 on pathways leading to metabolic complications remains unclear in humans. Pharmacological inhibition of 11HSD1 has been achieved in liver with carbenoxolone, which enhances hepatic insulin sensitivity. | ||
Visceral obesity may be secondary to enhanced local activation of cortisol via increased levels and activity of 11β-HSD-1 in adipose tissue that result in abnormally high levels of cortisol in adipose tissue. Obesity is distinct from Cushing's syndrome in that the source of the elevated glucocorticoids is adipose tissue rather than the [[adrenal cortex]]. | Visceral obesity may be secondary to enhanced local activation of cortisol via increased levels and activity of 11β-HSD-1 in adipose tissue that result in abnormally high levels of cortisol in adipose tissue. Obesity is distinct from Cushing's syndrome in that the source of the elevated glucocorticoids is adipose tissue rather than the [[adrenal cortex]]. | ||
| Line 59: | Line 47: | ||
==Causes of type 2 diabetes in obese patients== | ==Causes of type 2 diabetes in obese patients== | ||
===Endoplasmic reticulum stress === | ===Endoplasmic reticulum stress === | ||
[[Endoplasmic reticulum stress]] (ER stress) is a molecular-level link connecting obesity, insulin resistance, and type 2 diabetes. | [[Endoplasmic reticulum stress]] (ER stress) is a molecular-level link connecting obesity, insulin resistance, and type 2 diabetes. Mice lacking X-box-binding protein-1 (XBP-1), a transcription factor used to modulate the body’s response to ER stress, as well as mice that had induced ER stress via pharmacological means, showed the development of insulin resistance. ER stress or down-regulation of XBP-1 causes the suppression of insulin receptor signaling in the body’s cells via activation of Jun kinases (JNKs). In mice, this insulin receptor suppression leads to increased insulin resistance and the development of type 2 diabetes. It is thought that increased activity of JNKs causes the phosphorylation of insulin receptor substrates (IRSs) within important tissues such as liver, muscle and fat. As well as insulin resistance, JNK activity can result in the inhibition of insulin production in pancreatic β-cells. In mice which lack JNKs such as JNK1, obesity-induced obesity prevalence is reduced, and in general they also have reduced adiposity. | ||
In summary, there is a key process which controls the detection of obesity-induced ER stress, causing an inhibition of insulin action that | In summary, there is a key process which controls the detection of obesity-induced ER stress, causing an inhibition of insulin action that leads to insulin resistance and T2DM. It is thought that ER stress is a precursor to cell inflammation as a result of obesity. This then leads to complete breakdown of glucose homeostasis. | ||
===Dysfunction of the pancreatic β-cells=== | ===Dysfunction of the pancreatic β-cells=== | ||
Many studies have shown the importance of insulin secretory capability in the formation of T2DM. If insufficient insulin is secreted by the pancreatic β-cells, then adequate glucose uptake cannot occur. Couple this with increased cell IR correlated to levels of obesity, and you have the root cause of why incidence of T2DM increases with increasing levels of obesity. <br /> | Many studies have shown the importance of insulin secretory capability in the formation of T2DM. If insufficient insulin is secreted by the pancreatic β-cells, then adequate glucose uptake cannot occur. Couple this with increased cell IR correlated to levels of obesity, and you have the root cause of why incidence of T2DM increases with increasing levels of obesity. <br /> | ||
In mice fed | In mice fed a high fat diet, subsequent T2DM is at least party due to reduced insulin secretion in response to greater IR. Analysis of insulin secretion from isolated pancreatic islets of these mice found dysfunction in the islets for the production and/or secretion of insulin. Average islet insulin levels in HFD mice were found to be significantly lower than a control group. In addition, the islets from the HFD mice showed significantly lower insulin secretion than the control group. An increase in glucagon-positive cells within the islets was also discovered in the HFD group. These physiological changes are all present in human cases of T2DM. | ||
It is also possible that there is increased pancreatic β-cell apoptosis, induced by increasing | It is also possible that there is increased pancreatic β-cell apoptosis, induced by increasing obesity, reducing the level of insulin secretion. This reduced insulin secretion cannot then cope with the increasing cell IR caused by obesity. Further research has shown that dysfunction and death of the pancreatic β-cells may be as a result of cell inflammation due to hyperglycemia, dyslipidemia and increased levels of adipokines. | ||
===Resistin=== | ===Resistin=== | ||
The discovery of [[resistin]] came about through the development of a new class of anti-diabetic drugs called [[thiazolidinediones]] (TZDs). These act by increasing a target tissue’s sensitivity to insulin. They are ligands for a nuclear receptor called [[peroxisome proliferator activated receptor-ϒ ]] (PPARϒ) which is found in abundance in adipocytes. Tests showed a high correlation between TZD/PPARϒ binding and glucose lowering ''in vivo''. However, the target genes of TZD-bound PPARϒ are unknown. To try and discover whether IR might be controlled by a TZD-controlled, adipocyte-originating substance, a genetic screen was carried out for genes induced by adipocyte formation but downregulated when treated with TZDs. This | The discovery of [[resistin]] came about through the development of a new class of anti-diabetic drugs called [[thiazolidinediones]] (TZDs). These act by increasing a target tissue’s sensitivity to insulin. They are ligands for a nuclear receptor called [[peroxisome proliferator activated receptor-ϒ ]] (PPARϒ) which is found in abundance in adipocytes. Tests showed a high correlation between TZD/PPARϒ binding and glucose lowering ''in vivo''. However, the target genes of TZD-bound PPARϒ are unknown. To try and discover whether IR might be controlled by a TZD-controlled, adipocyte-originating substance, a genetic screen was carried out for genes induced by adipocyte formation but downregulated when treated with TZDs. This produced evidence of a TZD-regulated protein, called ''resistin''. | ||
Resistin | Resistin expression increases when adipocytes differentiate, and decreases with administration of TZD drugs such as [[rosiglitazone]], [[pioglitazone]] and [[troglitazon]]. In mice, resistin gene is expressed almost exclusively in white adipose tissue, with highest expression in female gonadal fat. An amino acid sequence expressed in humans with a large similarity to resistin was also found. In mice, serum levels of resistin decrease with fasting and are restored with re-feeding. In mice fed on a 45% fat content diet for 8 weeks, the levels of resistin in serum are greatly elevated, initially increasing within four weeks of the diet being adopted, the same point as when the mice become obese and insulin resistant. Higher than normal resistin levels can also be detected in leptin-deficient (''ob/ob'') mice and in leptin receptor deficient (''db/db'') mice, both of which are genetically predisposed to obesity and T2DM. | ||
Intraperitoneal administration of resistin to mice results in impaired insulin sensitivity, while insulin levels remain normal. Both ''in vitro'' and ''in vivo'' studies show that neutralization of resistin causes enhanced insulin action and glucose uptake. In obese, diabetic mice, resistin neutralization causes reduced levels of hyperglycemia by increasing insulin sensitivity. | Intraperitoneal administration of resistin to mice results in impaired insulin sensitivity, while insulin levels remain normal. Both ''in vitro'' and ''in vivo'' studies show that neutralization of resistin causes enhanced insulin action and glucose uptake. In obese, diabetic mice, resistin neutralization causes reduced levels of hyperglycemia by increasing insulin sensitivity. | ||
| Line 80: | Line 68: | ||
==The immunology of obesity== | ==The immunology of obesity== | ||
T2DM has long been considered primarily a metabolic disease. A series of recent studies have challenged this dogma and implicated an unlikely candidate system in the promotion of disease onset - the immune system. Mild inflammation of fat tissue in obese patients reportedly acts through immune-cell processes to impair insulin signalling in adipocytes. This work therefore provides a novel way of understanding the link between obesity and T2DM. | T2DM has long been considered primarily a metabolic disease. A series of recent studies have challenged this dogma and implicated an unlikely candidate system in the promotion of disease onset - the immune system. Mild inflammation of fat tissue in obese patients reportedly acts through immune-cell processes to impair insulin signalling in adipocytes. This work therefore provides a novel way of understanding the link between obesity and T2DM. | ||
Adipocytes | [[Adipocytes]] have a dual role as both a fat storage depot and an endocrine organ. Obesity impairs the performance of adipose tissue and can induce a state of chronic, low-grade inflammation (Feuerer et al., 2009). However, unlike other forms of inflammation, fat inflammation appears to escape immune regulation. The factors that initiate this inflammatory cascade are poorly understood. Depending on its state, adipose tissue will activate various phenotypes of T-cells (‘non-obese’ CD4 or ‘obese’ CD8), which in turn regulate (or fail to regulate) the infiltration of macrophages. It is this permeation of macrophages and their production of proinflammatory cytokines that results in chronic inflammation. The induction of chronic inflammation impairs insulin signalling in the adipocytes, which in turn leads to lipolysis and the release of non-esterified fatty acids (NEFAs) into the circulation. These fatty acids induce IR in the liver and skeletal muscles and impair B-cell function. Several groups have recently targeted the different T-cell populations and both reversed and prevented the onset of obesity-induced T2DM. | ||
Depending on its state, adipose tissue will activate various phenotypes of T-cells (‘non-obese’ CD4 or ‘obese’ CD8), which in turn regulate (or fail to regulate) the infiltration of macrophages. It is this permeation of macrophages and their production of proinflammatory cytokines that results in chronic inflammation. The induction of chronic inflammation impairs insulin signalling in the adipocytes, which in turn leads to lipolysis and the release of non-esterified fatty acids (NEFAs) into the circulation. These fatty acids induce IR in the liver and skeletal muscles and impair B-cell function. Several groups have recently targeted the different T-cell populations and both reversed and prevented the onset of obesity-induced T2DM. | |||
Feuerer et al. (2009) isolated a specific phenotype of T-cell, CD4, which is enriched in the adipose tissue of lean mice but dramatically reduced in that of obese, insulin-resistant mice. Through loss-of-function experiments it was shown that CD4 cells are functionally active and their absence results in inflammatory cytokine production and reduced glucose uptake. Importantly, CD4 T-cells were only shown to behave in this manner in visceral fat stores, which, unlike subcutaneous stores, are associated with the development of T2DM. A complementary study, performed by Winer et al. (2009), demonstrated that CD4 T-cell transfer reverses weight gain and IR in null mutants. Nevertheless, because the mice lost weight after the CD4 T-cell transfer, it makes conclusive interpretation of the data difficult. This data led the authors to conclude that obesity-associated metabolic abnormalities are under the pathophysiological control of CD4 T-cells, which inversely control the infiltration of problematic macrophages . | Feuerer et al. (2009) isolated a specific phenotype of T-cell, CD4, which is enriched in the adipose tissue of lean mice but dramatically reduced in that of obese, insulin-resistant mice. Through loss-of-function experiments it was shown that CD4 cells are functionally active and their absence results in inflammatory cytokine production and reduced glucose uptake. Importantly, CD4 T-cells were only shown to behave in this manner in visceral fat stores, which, unlike subcutaneous stores, are associated with the development of T2DM. A complementary study, performed by Winer et al. (2009), demonstrated that CD4 T-cell transfer reverses weight gain and IR in null mutants. Nevertheless, because the mice lost weight after the CD4 T-cell transfer, it makes conclusive interpretation of the data difficult. This data led the authors to conclude that obesity-associated metabolic abnormalities are under the pathophysiological control of CD4 T-cells, which inversely control the infiltration of problematic macrophages . | ||
| Line 94: | Line 79: | ||
Both of these drugs are already used to treat other medical conditions and are therefore both safe and available, however the question that remains to be answered is do Zaditor and cromolyn offer similar protection against diabetes in humans? At present the application from model to human appears positive. A study into T-cell concentration in human abdominal fat tissue by Winer et al. (2009) has revealed an abundance of protective CD4 T-cells in normal weight individuals when compared to that of obese, diabetic patients, as well a reflection of inverse number of macrophages. | Both of these drugs are already used to treat other medical conditions and are therefore both safe and available, however the question that remains to be answered is do Zaditor and cromolyn offer similar protection against diabetes in humans? At present the application from model to human appears positive. A study into T-cell concentration in human abdominal fat tissue by Winer et al. (2009) has revealed an abundance of protective CD4 T-cells in normal weight individuals when compared to that of obese, diabetic patients, as well a reflection of inverse number of macrophages. | ||
==Treatment of obesity and type 2 diabetes== | ==Treatment of obesity and type 2 diabetes== | ||
| Line 115: | Line 89: | ||
There is evidence that the development of T2DM can be prevented or delayed through the instigation of lifestyle modification and drugs such as metformin and orlistat. Physical exercise and weight loss are among the most effective methods for preventing the onset of diabetes, and a large randomised study concluded that lifestyle intervention was more effective that metformin. However lifestyle modification is often found to be difficult to sustain by obese patients. <ref>Jermendy G (2005) Can type 2 diabetes mellitus be considered preventable? ''Diabetess res clin pr'' 68:S73-S81</ref><ref>Diabetes Prevention Program Research Group(2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin ''N Engl J Med'' 346:393–403</ref> | There is evidence that the development of T2DM can be prevented or delayed through the instigation of lifestyle modification and drugs such as metformin and orlistat. Physical exercise and weight loss are among the most effective methods for preventing the onset of diabetes, and a large randomised study concluded that lifestyle intervention was more effective that metformin. However lifestyle modification is often found to be difficult to sustain by obese patients. <ref>Jermendy G (2005) Can type 2 diabetes mellitus be considered preventable? ''Diabetess res clin pr'' 68:S73-S81</ref><ref>Diabetes Prevention Program Research Group(2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin ''N Engl J Med'' 346:393–403</ref> | ||
When treating T2DM, main aims are to return metabolic disturbances to normal, achieve good glycemic control and assist with weight management. | When treating T2DM, main aims are to return metabolic disturbances to normal, achieve good glycemic control and assist with weight management. Dietary management in diabetic patients is particularly important, in order to reduce the cardiovascular risks associated with central obesity. Patients with T2DM need to restrict carbohydrate and total calorific intake and eat foods of low glycemic index, to reduce the post prandial rise in blood glucose. When dietary management is not successful, pharmacological intervention is added, including anti-diabetic drugs to prevent hyperglycaemia, ACE inhibitors to treat hypertension and statins or fibrates to treat hyperlipidaemia.<ref>Lean MEJ ''et al.'' (1990) Obesity, weight loss and prognosis in type 2 diabetes. ''Diabetic Med'' 7:228-233</ref> | ||
'''Metformin''' is recommended as first-line treatment in T2DM patients. When this fails, other agents are added to provide combination therapy. The majority of T2DM patients require combination therapy, because monotherapy with metformin usually only maintains good metabolic control in the short term. Treatments which can be added include sulphonylureas, acarbose, glucagon-like peptide-1 (GLP-1) analogues, thiazolidinediones, glinides, or insulin. <ref>Monami M ''et al.'' (2008) Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. ''Diabetes Res Clin Pr'' 79:196–203</ref> | '''Metformin''' is recommended as first-line treatment in T2DM patients. When this fails, other agents are added to provide combination therapy. The majority of T2DM patients require combination therapy, because monotherapy with metformin usually only maintains good metabolic control in the short term. Treatments which can be added include sulphonylureas, acarbose, glucagon-like peptide-1 (GLP-1) analogues, thiazolidinediones, glinides, or insulin. <ref>Monami M ''et al.'' (2008) Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. ''Diabetes Res Clin Pr'' 79:196–203</ref> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 11:24, 10 October 2010
The term diabesity was coined by Ethan Sims in 1973, to describe the close relationship between type 2 diabetes (T2DM) and obesity. Their findings suggested that by overfeeding young men, with no previous family history of diabetes, the initial signs of diabetes were induced. This excess consuption led to increases in insulin production, plasma glucose, triglycerides and eventually impaired glucose tolerance; all signs predisposing one to T2DM and obesity[1][2]
Diabetes mellitus type 2 is a disorder where cells fail to take up glucose from the blood. Glucose is the fuel for respiration which produces energy for our cells to function properly. As there is no glucose entering the cells, they waste away and are forced to consume their own proteins. Diabetes is the foremost cause of kidney failure (diabetic nephropathy), blindness (diabetic retinopathy), and amputation in adults (diabetic neuropathy). People with this disease lack the ability to utilize the hormone insulin. Insulin is produced by the pancreas after a meal inresponse to increased concentrations of glucose in the blood. The insulin signal attaches to specific receptors on the surface of target cells, causing them to switch on their glucose-transporting machinery. Type 2 diabetics have normal or even elevated levels of insulin in their blood, and normal insulin receptors, but the binding of insulin to its receptors does not turn on the glucose-transporting machinery.
Proteins called IRS proteins (insulin receptor substrate) bind with the insulin receptor inside the cell. The receptor responds by adding a phosphate group onto the IRS molecules. This rouses the IRS molecules into action, and they activate a variety of processes, including an enzyme that turns on the glucose transporter machinery. When the IRS genes are deliberately inactivated in transgenic “knockout” mice, type 2 diabetes results. However, there are no IRS gene mutations in inherited type 2 diabetics; the IRS genes are normal. This suggests that in type 2 diabetes something is impeding with the action of the IRS proteins. An estimated 80% of those who develop type 2 diabetes are obese, an enticing association.
The Genetics of Diabesity
Thrifty gene hypothesis
The thrifty gene hypothesis remains a popular explanation for the growing rise of diabetes and obesity. In 1962, [3] Neel proposed this hypothesis to explain the high prevalence of T2D in recently westernized nations. He suggested that the “thrifty” genes that predispose to T2D and obesity are evolutionary advantageous. He proposed that in the primitive age, where famine and food shortages would be common, individuals with “thrifty genes” were more likely to survive. This is because these individuals could store a high percentage of their energy as fat, and use this energy store to survive times of famine. However, in the modern world there is a constant abundance of food; the thrifty genes prepare us for a famine that never comes, resulting in a huge rise in T2D and obesity [4] Those from ethnic populations who had experienced famine and poor nutrition until relatively recently, are shown to have the highest rates in the world (3) [5]For example, research on the Pima Indians has contributed in providing clear evidence that a sudden abundance in food can lead to a substantial rise in obesity rates as well as T2D rates. The research found that 50% of adult Pima Indians have diabetes, and 95% of these were overweight. [6]
Visceral fat accumulation and type 2 diabetes
Excess visceral adipose tissue increases the risk for type 2 diabetes. Recently it has been discovered that excess fat within the abdomen, known as visceral adiposity, creates a serious independent health risk of metabolic complications than accumulation of adipose tissue in other regions. Visceral adiposity is related with an increase in insulin resistance, whereas abdominal subcutaneous fat is not. (Insulin resistance describes the impaired ability of insulin to suppress hepatic glucose output and promote glucose disposal in the periphery.) As diabetes gets worse, patients have higher blood sugar levels (hyperglycaemia) because their beta cells, in the pancreas, are unable to keep making enough insulin. In insulin resistance, normal amounts of insulin are unable to produce a normal response from adipose, muscle and liver cells.
In 1994, a new hormone was found, called leptin, that provides feedback to the brain of the level of fat in the body. Leptin suppresses appetite, but most obese people have very high leptin levels, as leptin is secreted by adipose cells. Therefore, obesity is not generally caused by a deficiency in leptin; instead there seems to be a defect in leptin signalling.
Cnop et al showed that visceral fat is the best predictor of insulin sensitivity whilst subcutaneous fat establishes leptin levels [9].
Adipocytes produce an array of other peptides including adiponectin, resistin and TNF alpha 25. They are act on peripheral tissue and thereby affect insulin sensitivity and the processes involved in substrate metabolism. As discussed later, resistin could be one of the key connections between obesity and DM2 by inhibiting adipogenesis (formation of adipse tissue) and increasing the circulating free fatty acids. Adipose tissue is an important organ that can regulate energy balance through the complex control of hormone and neuronal signals using leptin, adiponectin, resistin, TNF alpha, and angiotensin.
The lipoprotein profile related to obesity and insulin resistance is mostly due to intra-abdominal fat [4]. There are better measures of obesity (particularly visceral obesity) that can predict diabetes. These include waist circumference, the waist-to-hip ratio and insulin resistance.
Fat cells show elevated hydrolysis of stored triglycerides and increased free fatty acids into the blood. Insulin resistance reduces the antilipolytic effect of insulin, which leads to reduced glucose uptake and increased release of free fatty acids and glycerol. [5]
Excess free fatty acids are taken by the portal vein to the liver. The liver is then overwhelmed by the free fatty acidsand starts up typical IR metabolic processes. The liver responds by increasing glyconeogenesis (production of glygogen), increasing triglyceride, apolipoprotein B and very low density lipoprotein (VLDL) production. This in turn increases the production of low density lipoproteins and the reduction of high density lipoproteins (HDLs). This lipid profile is known as atherogenic dyslipidaemia as it eventually leads to atherosclerosis.
Intramyocellular lipids (IMCL) are more closely associated to insulin resistance than to body mass index, waist-to-hip ratio, or total body fat. High free fatty acid and VLDL levels are a key cause of fat accumulation in muscle cells and IMCL increases have been seen in patients with insulin resistance. Surgical removal of visceral fat had a positive effect on the hepatic and peripheral insulin sensitivity, and on leptin and TNFα levels. [6]
Long term exposure of beta cells to increased FA levels causes damaging effects such as increased insulin secretion at low glucose concentrations, decreased proinsulin production, exhaustion of insulin reserves and reduced response to concentrations of glucose stimulus. Solomon and Mayer [8] were first to associate glucocorticoids as a required factor in genetic obesity, observing that obesity was avoided after bilateral adrenalectomy and completely restored by cortisol replacement.
11β-Hydroxysteroid dehydrogenase type 1 (11HSD-1) catalyses the conversion of inactive cortisone to active cortisol, a potent glucocorticoid. It is found throughout the body and is a highly regulated enzyme that increases the ligand accessibility for glucocorticoid receptors.
Excessive glucocorticoid exposure causes central obesity, hypertension, and dyslipidaemia and insulin resistance, as seen in Cushing’s syndrome. Transgenic mice over-expressing 11HSD1 in white adipose tissue have these features as well, while 11HSD1 knockout mice are protected from these metabolic abnormalities. In human idiopathic obesity, circulating cortisol levels are not elevated but 11HSD1 mRNA and activity is increased in subcutaneous adipose. The impact of increased adipose 11HSD1 on pathways leading to metabolic complications remains unclear in humans. Pharmacological inhibition of 11HSD1 has been achieved in liver with carbenoxolone, which enhances hepatic insulin sensitivity.
Visceral obesity may be secondary to enhanced local activation of cortisol via increased levels and activity of 11β-HSD-1 in adipose tissue that result in abnormally high levels of cortisol in adipose tissue. Obesity is distinct from Cushing's syndrome in that the source of the elevated glucocorticoids is adipose tissue rather than the adrenal cortex.
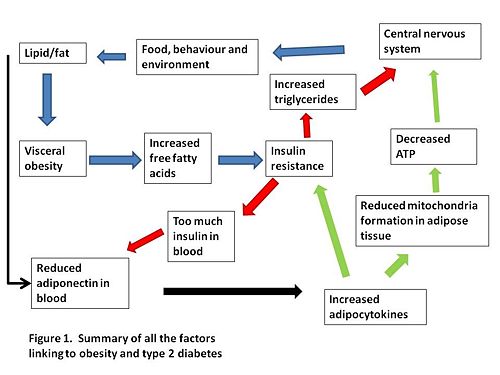
Links between environmental influences, the layout of visceral fat, the PPARs, the adiponectin and the adipocytokines still need to be completely clarified. A summary of how we think visceral obesity causes insulin resistance and type 2 diabetes is shown in figure 1.
Causes of type 2 diabetes in obese patients
Endoplasmic reticulum stress
Endoplasmic reticulum stress (ER stress) is a molecular-level link connecting obesity, insulin resistance, and type 2 diabetes. Mice lacking X-box-binding protein-1 (XBP-1), a transcription factor used to modulate the body’s response to ER stress, as well as mice that had induced ER stress via pharmacological means, showed the development of insulin resistance. ER stress or down-regulation of XBP-1 causes the suppression of insulin receptor signaling in the body’s cells via activation of Jun kinases (JNKs). In mice, this insulin receptor suppression leads to increased insulin resistance and the development of type 2 diabetes. It is thought that increased activity of JNKs causes the phosphorylation of insulin receptor substrates (IRSs) within important tissues such as liver, muscle and fat. As well as insulin resistance, JNK activity can result in the inhibition of insulin production in pancreatic β-cells. In mice which lack JNKs such as JNK1, obesity-induced obesity prevalence is reduced, and in general they also have reduced adiposity.
In summary, there is a key process which controls the detection of obesity-induced ER stress, causing an inhibition of insulin action that leads to insulin resistance and T2DM. It is thought that ER stress is a precursor to cell inflammation as a result of obesity. This then leads to complete breakdown of glucose homeostasis.
Dysfunction of the pancreatic β-cells
Many studies have shown the importance of insulin secretory capability in the formation of T2DM. If insufficient insulin is secreted by the pancreatic β-cells, then adequate glucose uptake cannot occur. Couple this with increased cell IR correlated to levels of obesity, and you have the root cause of why incidence of T2DM increases with increasing levels of obesity.
In mice fed a high fat diet, subsequent T2DM is at least party due to reduced insulin secretion in response to greater IR. Analysis of insulin secretion from isolated pancreatic islets of these mice found dysfunction in the islets for the production and/or secretion of insulin. Average islet insulin levels in HFD mice were found to be significantly lower than a control group. In addition, the islets from the HFD mice showed significantly lower insulin secretion than the control group. An increase in glucagon-positive cells within the islets was also discovered in the HFD group. These physiological changes are all present in human cases of T2DM.
It is also possible that there is increased pancreatic β-cell apoptosis, induced by increasing obesity, reducing the level of insulin secretion. This reduced insulin secretion cannot then cope with the increasing cell IR caused by obesity. Further research has shown that dysfunction and death of the pancreatic β-cells may be as a result of cell inflammation due to hyperglycemia, dyslipidemia and increased levels of adipokines.
Resistin
The discovery of resistin came about through the development of a new class of anti-diabetic drugs called thiazolidinediones (TZDs). These act by increasing a target tissue’s sensitivity to insulin. They are ligands for a nuclear receptor called peroxisome proliferator activated receptor-ϒ (PPARϒ) which is found in abundance in adipocytes. Tests showed a high correlation between TZD/PPARϒ binding and glucose lowering in vivo. However, the target genes of TZD-bound PPARϒ are unknown. To try and discover whether IR might be controlled by a TZD-controlled, adipocyte-originating substance, a genetic screen was carried out for genes induced by adipocyte formation but downregulated when treated with TZDs. This produced evidence of a TZD-regulated protein, called resistin.
Resistin expression increases when adipocytes differentiate, and decreases with administration of TZD drugs such as rosiglitazone, pioglitazone and troglitazon. In mice, resistin gene is expressed almost exclusively in white adipose tissue, with highest expression in female gonadal fat. An amino acid sequence expressed in humans with a large similarity to resistin was also found. In mice, serum levels of resistin decrease with fasting and are restored with re-feeding. In mice fed on a 45% fat content diet for 8 weeks, the levels of resistin in serum are greatly elevated, initially increasing within four weeks of the diet being adopted, the same point as when the mice become obese and insulin resistant. Higher than normal resistin levels can also be detected in leptin-deficient (ob/ob) mice and in leptin receptor deficient (db/db) mice, both of which are genetically predisposed to obesity and T2DM.
Intraperitoneal administration of resistin to mice results in impaired insulin sensitivity, while insulin levels remain normal. Both in vitro and in vivo studies show that neutralization of resistin causes enhanced insulin action and glucose uptake. In obese, diabetic mice, resistin neutralization causes reduced levels of hyperglycemia by increasing insulin sensitivity.
The molecular target of resistin is unknown, but it is hypothesized that it modulates at least one step in the insulin signaling pathway. At present it is unclear whether levels of resistin have a major effect on insulin activity in humans. In humans, resistin is thought to be secreted by macrophages not adipocytes. Despite this, there is still a strong correlation in humans between high levels of resistin, obesity, and T2DM.
The immunology of obesity
T2DM has long been considered primarily a metabolic disease. A series of recent studies have challenged this dogma and implicated an unlikely candidate system in the promotion of disease onset - the immune system. Mild inflammation of fat tissue in obese patients reportedly acts through immune-cell processes to impair insulin signalling in adipocytes. This work therefore provides a novel way of understanding the link between obesity and T2DM.
Adipocytes have a dual role as both a fat storage depot and an endocrine organ. Obesity impairs the performance of adipose tissue and can induce a state of chronic, low-grade inflammation (Feuerer et al., 2009). However, unlike other forms of inflammation, fat inflammation appears to escape immune regulation. The factors that initiate this inflammatory cascade are poorly understood. Depending on its state, adipose tissue will activate various phenotypes of T-cells (‘non-obese’ CD4 or ‘obese’ CD8), which in turn regulate (or fail to regulate) the infiltration of macrophages. It is this permeation of macrophages and their production of proinflammatory cytokines that results in chronic inflammation. The induction of chronic inflammation impairs insulin signalling in the adipocytes, which in turn leads to lipolysis and the release of non-esterified fatty acids (NEFAs) into the circulation. These fatty acids induce IR in the liver and skeletal muscles and impair B-cell function. Several groups have recently targeted the different T-cell populations and both reversed and prevented the onset of obesity-induced T2DM.
Feuerer et al. (2009) isolated a specific phenotype of T-cell, CD4, which is enriched in the adipose tissue of lean mice but dramatically reduced in that of obese, insulin-resistant mice. Through loss-of-function experiments it was shown that CD4 cells are functionally active and their absence results in inflammatory cytokine production and reduced glucose uptake. Importantly, CD4 T-cells were only shown to behave in this manner in visceral fat stores, which, unlike subcutaneous stores, are associated with the development of T2DM. A complementary study, performed by Winer et al. (2009), demonstrated that CD4 T-cell transfer reverses weight gain and IR in null mutants. Nevertheless, because the mice lost weight after the CD4 T-cell transfer, it makes conclusive interpretation of the data difficult. This data led the authors to conclude that obesity-associated metabolic abnormalities are under the pathophysiological control of CD4 T-cells, which inversely control the infiltration of problematic macrophages .
A separate study by Nishimura et al. (2009) revealed that a different type of T-cell, CD8, are increased in obese mice and precede chronic inflammation observed in adipose tissue. Similarly, the adoptive transfer of CD8 T-cells resulted in adipose inflammation. Together this evidence led the authors to propose that obese adipose tissue activates CD8+ T-cells, which drive the recruitment of macrophages and their differentiation into an inflammatory rather than anti-inflammatory phenotype.
Another aspect of the immune system that has been implicated in T2DM onset is mast cell function. Mast cells respond to allergic and parasitic challenge by releasing inflammatory mediators, thus playing an integral protective role. An excess of mast cells beyond that of what is immunologically necessary can lead to mast cell instability and inflammation. Shi et al. observed that the white adipose tissue of obese mice possesses a significantly greater number of mast cells when compared to that of lean equivalents. This led the authors to ask whether the manipulation of mast cell number, achieved through genetic reduction and pharmacological equalization, can reduce the onset of obesity and T2DM. In the first set of experiments, genetically mast cell-deficient mice and control mice were fed on a Western-diet for three months. Loss of mast cell function appeared to be having the effect of lowering serum leptin, increasing glucose tolerance and increasing insulin sensitivity in comparison to the control group. In the second strand of experimentation, mice were treated with mast cell-stabilizing medication to ask whether diet-induced obesity and diabetes could be inhibited. After two months on a Western-diet, mice were either switched to a healthy diet, supplied with mast-cell stabilizing medication, or a combination of both. While the dietary adjustment caused minor improvements, the medication stimulated significant restitution and the combination of both allowed a near full recovery in comparison to control group who continued on a Western-diet.
Both of these drugs are already used to treat other medical conditions and are therefore both safe and available, however the question that remains to be answered is do Zaditor and cromolyn offer similar protection against diabetes in humans? At present the application from model to human appears positive. A study into T-cell concentration in human abdominal fat tissue by Winer et al. (2009) has revealed an abundance of protective CD4 T-cells in normal weight individuals when compared to that of obese, diabetic patients, as well a reflection of inverse number of macrophages.
Treatment of obesity and type 2 diabetes
Obesity, alongside genetic predisposition, is one of the most significant risk factors for the development of T2DM. Lifestyle changes provide the basis of treatment in all obese patients. When lifestyle changes fail to reduce the weight in obese patients, anti-obesity drugs are used. [1] There are few well-tolerated drugs which have been proven to have long term efficacy in maintaining weight loss. Current available medications include sibutramine and orlistat.
Sibutramine reduces body weight and appetite and increases satiety. Numerous prospective randomised controlled trials have shown it to be effective, with one trial finding that patients on sibutramine lost 4.3kg or 4.6% more weight than those taking the placebo. The most common adverse effects are dry mouth, constipation and insomnia. Orlistat acts by inhibiting pancreatic and gastrointestinal lipases, preventing absorption of about 30% of dietary fat. Randomized controlled trials have shown that patients taking this have lost 2.7kg or 2.9% more weight than controls. As orlistat reduces LDL and cholesterol levels independently of reductions in body weight, it also retards the progression to a diabetic state and aids glycemic control in patients with diabetes. Side effects include fecal urgency and abdominal cramping.[<re>Chaputy JP, Tremblay A (2006) Current and novel approaches to the drug therapy of obesity. Eur J Clin Pharmacol 62 </ref>
Patients with impaired glucose tolerance, impaired fasting glucose and obesity are all at a high risk of developing type T2DM, therefore combination therapy for glycaemic control and weight management is often required. Several strategies are used, including the promotion of weight loss through lifestyle modifications and anti-obesity drugs, improving glycemic control through the reduction of IR and the treatment of common associated risk factors such as hypertension and dyslipidaemia to improve cardiovascular prognosis [7].
There is evidence that the development of T2DM can be prevented or delayed through the instigation of lifestyle modification and drugs such as metformin and orlistat. Physical exercise and weight loss are among the most effective methods for preventing the onset of diabetes, and a large randomised study concluded that lifestyle intervention was more effective that metformin. However lifestyle modification is often found to be difficult to sustain by obese patients. [8][9]
When treating T2DM, main aims are to return metabolic disturbances to normal, achieve good glycemic control and assist with weight management. Dietary management in diabetic patients is particularly important, in order to reduce the cardiovascular risks associated with central obesity. Patients with T2DM need to restrict carbohydrate and total calorific intake and eat foods of low glycemic index, to reduce the post prandial rise in blood glucose. When dietary management is not successful, pharmacological intervention is added, including anti-diabetic drugs to prevent hyperglycaemia, ACE inhibitors to treat hypertension and statins or fibrates to treat hyperlipidaemia.[10]
Metformin is recommended as first-line treatment in T2DM patients. When this fails, other agents are added to provide combination therapy. The majority of T2DM patients require combination therapy, because monotherapy with metformin usually only maintains good metabolic control in the short term. Treatments which can be added include sulphonylureas, acarbose, glucagon-like peptide-1 (GLP-1) analogues, thiazolidinediones, glinides, or insulin. [11]
References
- ↑ Sims EAH et al. (1973) Endocrine and metabolic effects of experimental obesity in man, Recent Prog Horm Res 29:457–96
- ↑ Haslam DW, James WP (2005) ObesityLancet 366:1197–209
- ↑ The original paper that proposes the thrifty genotype. Neel JV (1962) Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"?. Am J Hum Genet 14:353–62
- ↑ Joffe B, Zimmet P (1998) The thrifty genotype in type 2 diabetes. Endocrine 9:139–41
- ↑ Relates genetic and environmental factors. Thrifty gene hypothesis summarised Noel Cameron, Nicholos G Norgan, George Ellison, Childhood Obesity Contemporary Issues 2006, CRC press, Taylor and Francis Group.
- ↑ Website which describes the study on Pima Indians - rates of Obesity and Diabetes http://diabetes.niddk.nih.gov/DM/pubs/pima/obesity/obesity.htm
- ↑ ScheenAJ (2000) Treatment of diabetes in patients with severe obesity. Biomed Pharmacother 54:74-79
- ↑ Jermendy G (2005) Can type 2 diabetes mellitus be considered preventable? Diabetess res clin pr 68:S73-S81
- ↑ Diabetes Prevention Program Research Group(2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin N Engl J Med 346:393–403
- ↑ Lean MEJ et al. (1990) Obesity, weight loss and prognosis in type 2 diabetes. Diabetic Med 7:228-233
- ↑ Monami M et al. (2008) Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pr 79:196–203