Nuclear chemistry
Nuclear chemistry is a subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties. It can be divided into several main categories:
| Mark Rust has nominated Chemistry/Draft&oldid=100012889 this version of this article for approval. Other editors may also sign to support approval. The Chemistry Workgroup is overseeing this approval. Unless this notice is removed, the article will be approved on December 31, 2006. |
Early history
After the discovery of X-rays by Wilhelm Röntgen, many scientists began to work on ionizing radiation. One of these was Henri Becquerel, who was investigating the relationship between phosphorescence and the blackening of photographic plates. When Becquerel (working in France) discovered that, with no external source of energy, the uranium generated rays which could blacken (or fog) the photographic plate, radioactivity was discovered. Marie Curie (working in Paris) and her husband Pierre Curie isolated two new radioactive elements from uranium ore, they used radiometric methods to identify which stream the radioactivity was in after each each chemical separation. They separated the uranium ore into each of the different chemical elements that were known at the time, and measured the radioactivity of each fraction. They then attempted to separate these radioactive fractions further, to isolate a smaller fraction with a higher specific activity (radioactivity divided by mass). In this way, they isolated polonium and radium. Their daughter Irène Joliot-Curie and her husband were the first to 'create' radioactivity: they bombarded boron with alpha particles to make a proton-rich isotope of nitrogen; this isotope emitted positrons.[5] In addition, they bombarded aluminium and magnesium with neutrons to make new radioisotopes.
Ernest Rutherford, working in Canada and England, discovered that radioactivity decays according to first order kinetics (half life); he coined the terms alpha, beta and gamma rays. He also converted nitrogen into oxygen, and most importantly he supervised the students who did the Geiger-Marsden experiment (gold leaf experiment) which showed that the 'plum pudding model' of the atom was wrong. In the plum pudding model, proposed by J. J. Thomson in 1904, the atom is composed of electrons surrounded by a 'cloud' of positive charge to balance the electrons' negative charge. To Rutherford, the results of the gold foil experiment implied that the positive charge was confined to a very small nucleus leading first to the Rutherford model, and eventually to the Bohr model of the atom.
Main areas
Radiochemistry
Radiochemistry is the chemistry of radioactive materials. In radiochemistry, radioactive isotopes of elements are used to study the properties and chemical reactions of ordinary non-radioactive isotopes (often within radiochemistry the absence of radioactivity leads to a substance being described as being inactive as the isotopes are stable). An example of a biological use of radiochemistry is the study of DNA using radioactive phosphorus-32.
Radiation chemistry
Radiation chemistry is the study of the chemical effects of radiation on matter; this is very different to radiochemistry as no radioactivity needs to be present in the material which is being chemically changed as a result of the radiation. An example of radiation chemistry is the conversion of water into hydrogen gas and hydrogen peroxide.
Study of nuclear reactions
see also nuclear physics
A combination of radiochemistry and radiation chemistry is used to study nuclear reactions such as fission and fusion. For instance, some early evidence for nuclear fission was the formation of a shortlived radioisotope of barium which was isolated from neutron irradated uranium (Ba-139, with a half-life of 83 minutes and Ba-140, with a half-life of 12.8 days, are major fission products of uranium). At the time it was thought that this was a new radium isotope, as it was then standard radiochemical practise to use a barium sulphate carrier percipitate as a means of assisting in the isolation of radium.[6]. More recently, a combination of radiochemical methods and nuclear physics has been used to try to create new 'superheavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated.[1].
The nuclear fuel cycle
The chemistry associated with any part of the nuclear fuel cycle which includes nuclear reprocessing. The fuel cycle includes all the operations involved with the production of fuel from mining, ore processing, enrichment and fuel production (Front end of the cycle). Also it includes the 'in-pile' behaviour (use of the fuel in a reactor) before the back end of the cycle. The back end includes the management of the used nuclear fuel in either a cooling pond or dry storage, before it is either disposed of directly into an underground waste store or it is reprocessed.
The study of used fuel
Used nuclear fuel is studied in post irradation examination, where used fuel is examined to know more about the processes that occur in fuel during use, and how these might alter the outcome of an accident. For example, during normal use, the fuel expands due to thermal expansion. This causes cracking, and in extreme cases, such as during the power surge which destroyed the Chernobyl nuclear reactor in April, 1986, the fuel can shatter into very small fragments.
Fuel cladding interactions
The study of the nuclear fuel cycle includes both the study of the behaviour of nuclear materials both under normal conditions and under accident conditions. For example a large amount of work has been done on the interaction between uranium dioxide based fuel and the zirconium alloy tubing used to cover the fuel. In short using use the fuel swells due to thermal expansion and then starts to react with the surface of the zirconium alloy, to form a new layer which contains both fuel and zirconium (from the cladding). Then, on the fuel side of this mixed layer, is a layer of fuel which is a cesium rich layer which has a higher cesium to uranium ratio than most of the fuel. This is because xenon isotopes are formed as fission products and these diffuse out of the lattice of the fuel into voids such as the narrow gap between fuel and the cladding, after diffusing to these voids it decays to cesium isotopes. Because of the thermal gradient which exists in the fuel during use, the volatile fission products tend to be driven from the centre of the pellet to the rim area.[2]
Absorption of fission products on surfaces
Another important area of work within nuclear chemistry is the study of the interaction of fission products with surfaces. This is thought to control the rate of release and migration of fission products both from waste containers under normal conditions and from power reactors under accident conditions.
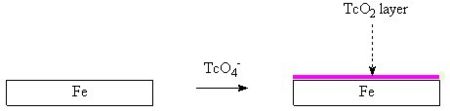
Tc
It is interesting to note that, like chromate and molybdate, the 99TcO4 anion can react with steel surfaces to form a corrosion resistant layer. In this way, these metaloxo anions act as anodic corrosion inhibitors. The formation of 99TcO2 on steel surfaces is one effect which will retard the release of 99Tc from nuclear waste drums and nuclear equipment which has become lost before decontamination (eg submarine reactors lost at sea). This 99TcO2 layer renders the steel surface passive, it inhibits the anodic corrosion reaction. It has also been shown that 99TcO4 anions react to form a layer on the surface of activated carbon (charcoal).
Iodine-131
In a similar way, the release of iodine-131 in a serious power reactor accident could be retarded by absorption on metal surfaces within the nuclear plant. A PhD thesis[7] was written on this subject at the Nuclear chemistry department[8] at Chalmers University of Technology in Sweden.[3]
Spinout areas
Some methods which were first developed within nuclear chemistry and physics have become so widly used within chemistry and other physical sciences that they may be best thought of as not being part of normal nuclear chemistry.
Isotopic chemistry
The area of Isotopic chemistry could be included in this subtopic, but due to the use of the isotope effect to investigate chemical mechanisms and the use of cosmogenic isotopes and long lived unstable isotopes in geology it is best to consider much of isotopic chemistry as being separate from nuclear chemistry.
Kinetics (use within mechanistic chemistry)
The mechanisms of chemical reactions can be investigated by observing the effect upon the kinetics of making an isotopic modification of a substrate in a reaction. This is now a standard method in organic chemistry, so it is best that the isotope effect is considered within the contex of organic chemistry. Briefly, the replacement of normal hydrogens (protons) within a chemical compound with deuterium causes the rate of molecular vibration (C-H, N-H and O-H bonds show this) to decrease, this then can lead to a decrease in the reaction rate if the rate determining step involves the breaking of a bond between hydrogen and another atom, hence if upon replacement of protons for deuteriums the reaction changes in rate it is reasonable to assume that the breaking of the bond to hydrogen is part of the step which determines the rate.
Uses within geology, biology and forensic science
Cosmogenic isotopes are formed by the interaction of cosmic rays with the nucleus of an atom. These can be used for dating purposes and for use as natural tracers. In addition, by careful measurement of some ratios of stable isotopes it is possible to obtain new insights into the origin of bullets, ages of ice samples, ages of rocks, and the diet of a person can be identified from a hair or other tissue sample. (See Isotope geochemistry and Isotopic signature for further details).
Nuclear magnetic resonance
Spectrscopy
NMR spectroscopy uses the net spin of nuclei in a substances upon energy absorption, and is used to identify molecules. This has now become a standard spectrscopic tool within synthetic chemistry, one of the main uses of NMR is as a means of determining the bond connectivity within an organic molecule.
Imaging
NMR imaging also uses the net spin of nuclei (commonly protons) as a means of imaging. This is commonly used in medicine because it can provide detailed images of the inside of a person without inflicting any radiation upon them. In a medical setting NMR is often known simply as "Magnetic resonance" imaging, as the word 'nuclear' has negative connotations for many people.
References
- ↑ For more details of the original discovery of nuclear fission see the work of Otto Hahn
- Meitner L, Frisch OR (1939) Disintegration of uranium by neutrons: a new type of nuclear reaction Nature 143:239-240 [1]
- ↑ Further reading on fuel cladding interactions: Tanaka K et al. (2006) Journal of Nuclear Materials 357:58-68
- ↑ Further reading on iodine absorption on metal surfaces
- Glänneskog H (2004) Interactions of I2 and CH3I with reactive metals under BWR severe-accident conditions, Nuclear Engineering and Design 227:323-9.
- Glänneskog H (2005) Iodine chemistry under severe accident conditions in a nuclear power reactor, PhD thesis, Chalmers University of Technology.
- A lot of other work on the iodine chemistry which would occur during a bad accident has been done.[2][3][4]
Text books
Radiochemistry and Nuclear Chemistry
- Choppin, Liljenenzin and Rydberg
- ISBN -0750674636, Butterworth-Heinemann, 2002
Description: Comprehensive textbook.
Radioactivity, Ionizing radiation and Nuclear Energy
- Hála and Navratil
- ISBN -807302053-X, Konvoj, 2003
Description: Basic textbook for undergraduates.
The Radiochemical Manual
- Edited by BJ Wilson
- Written by RJ Bayly, JR Catch, JC Charlton, CC Evans, TT Gorsuch, JC Maynard, LC Myerscough, GR Newbery, H Sheard, CBG Taylor and BJ Wilson.
- No ISBN number (too old for the ISBN system), The radiochemical centre (Amersham) was sold via HMSO, 1966 (second edition)
Description: Overview of the production and uses of sources, both open and sealed.