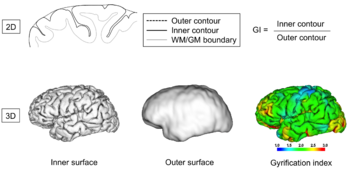
Gyrification
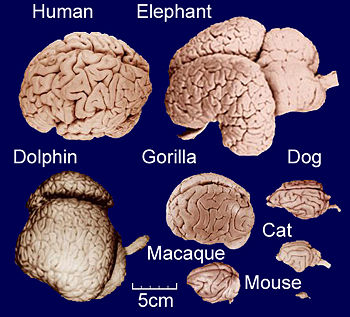
Comparative anatomy of adult brains from various vertebrate species, highlighting the differences in size and gyrification.
In the brain sciences, gyrification (or cortical folding, cortical convolution, fissuration or fissurization) refers to both the process and the extent of folding of the cerebral cortex in mammals as a consequence of brain growth during embryonic and early postnatal development.
In the process (also known as gyrogenesis), gyri (ridges) and sulci (grooves) form on the external surface of the brain (i.e. at the boundary between the cerebrospinal fluid and the gray matter)[1]. A low extent of gyrification in a given brain is commonly referred to as lissencephaly (which may range from agyria, the total absence of folding, to pachygyria[2]), while gyrencephaly describes a high degree of folding[3].
The term gyrification is also sometimes used instead of the more common term foliation[4] to describe the folding patterns of the vertebrate cerebellum[5] that is highly convoluted in other taxa, e.g. in birds[6], and of mushroom body calyces in insect brains[7].
Phylogeny
See also brain evolution.
As illustrated in the figure, gyrification occurs across mammals[8][9], with cetaceans dominating the upper end of the spectrum[10]. It generally increases slowly with overall brain size, following a power law [11]: Small-brained placental species are indeed lissencephalic[12][13], and amongst the two living species of monotremes, the small-brained platypus is lissencephalic, while the larger brains of echidna are gyrencephalic[14]. Conversely, large-brained mammals are usually highly gyrencephalic[15][16][17], with sirenians being a notable exception[18]. A range of theoretical models exist as to the degree to which gyrification hints at the evolution of cognitive abilities in a given range of species[19][20][21].
Ontogeny
See also brain development.
The folding process usually starts during fetal development—in humans around mid-gestation[22][1][23][24][25][26] —or shortly after birth, as in ferrets[27][28]. It proceeds synchronously in both hemispheres by an expansion of gyral tissue, while the sulcal roots remain in a relatively stable position throughout gyrogenesis[27][1][25]. In the adult human brain, variations due to gender[29], ethnicity[30] and age[31] have been demonstrated, and such interindividual differences appear to be highest in regions with strong gyrification[30].
Mechanism
While the extent of cortical folding has been found to be partly determined by genetic factors[32][33][34][35][36][37], the underlying biomechanical mechanisms are not yet well understood. The overall folding pattern, however, can be mechanistically explained in terms of the cerebral cortex buckling under the influence of non-isotropic forces[38][39][40][41][42]. Possible causes of the non-isotropy include differential growth of the cortical layers due to variations in the number and timing of cell divisions[43], cell migration, myelination, cortical connectivity and thalamic input[44], synaptic pruning, brain size and metabolism (phospholipids in particular), all of which may interact[45][46][47][48][3][49][50]. The folding, in turn, imposes constraints on the shape of cells, particulary in the outer cortical layers (V and VI)[51].
Function
The primary effect of a folding process is always an increase of surface area relative to volume. Due to the laminar arrangement of the cerebral cortex, an increased cerebral surface area correlates with an increased number of neurons, which is presumed to enhance the computational capacities of the cortex within some metabolic and connectivity limits[52]. In some areas of the human brain, gyrification appears indeed to reflect functional development[53] and thus to correlate with measures of intelligence[54], even though variations of these effects due to gender and age have been described [55].
Medical relevance
A number of disorders exist of which abnormal gyrification is a dominant feature, e.g. polymicrogyria or lissencephalic disorders[56] like agyria and pachygyria[57][58][59]. They usually occur bilaterally but cases of, e.g., unilateral lissencephaly, have been described[60]. Beyond these gross modifications of gyrification, more subtle variations occur in a number of neuropsychiatric disorders whose variety reflects the multitude of processes underlying gyrification[3]. Due to methodological advances in neuroimaging and computational morphometry since the late 1990s, folding patterns and abnormalities thereof can now be determined non-invasively. This is becoming increasingly important for clinical diagnostics, particular in relation to neuropsychiatric diseases like schizophrenia[61][62], autism[63], epilepsy[64], dyslexia[65], velocardiofacial syndrome[66], Attention deficit hyperactivity disorder (ADHD)[67] or Williams syndrome[68]. The direction of disease-associated changes depends on the cortical region and the disease subtype. In schizophrenics, for instance, gyrification has been found to increase in the dorsolateral prefrontal cortex[69] and, in different populations, to decrease in frontal and parietal regions of the left hemisphere[70]or even throughout both hemispheres[71].
Quantification
See also the Addendum.
From the perspective of brain morphometry, folding of a brain can be described in both local and global terms, once a suitable representation of a brain surface has been obtained from neuroimaging data by some surface extraction technique. The latter usually delivers a triangulated surface representing either the boundary between the cerebrospinal fluid and the gray matter or between the latter and the white matter but in principle, any surface in between would do as well (e.g. the central layer which is also sometimes used). Leaving the multiple issues of resolution and artifacts in these surface representations aside, the brain surface mesh, like any mesh of a closed three-dimensional manifold, can then be analyzed in terms of local curvature measures, from which global measures can be derived. Over the last decades, several such measures have been proposed[72][73]. Following the developments in imaging techniques, they were initially focused on quantification in two-dimensional spaces, later in three-dimensional ones.
References
- ↑ 1.0 1.1 1.2 Armstrong, E.; Schleicher, A.; Omran, H.; Curtis, M.; Zilles, K. (1995). "The Ontogeny of Human Gyrification". Cerebral Cortex 5 (1): 56-63.
- ↑ Dhellemmes, C.; S. Girard & O. Dulac et al. (1988), "Agyria—pachygyria and Miller-Dieker syndrome: clinical, genetic and chromosome studies", Human Genetics 79 (2): 163–167, DOI:10.1007/BF00280557
- ↑ 3.0 3.1 3.2 Francis, F.; G. Meyer & C. Fallet-Bianco et al. (2006), "Human disorders of cortical development: from past to present", European Journal of Neuroscience 23 (4): 877–893, DOI:10.1111/j.1460-9568.2006.04649.x
- ↑ Demaerel, P. (2002), "Abnormalities of cerebellar foliation and fissuration: classification, neurogenetics and clinicoradiological correlations", Neuroradiology 44 (8): 639–646, DOI:10.1007/s00234-002-0783-1
- ↑ Mares, V. & Z. Lodin (1970), "The cellular kinetics of the developing mouse cerebellum. II. The function of the external granular layer in the process of gyrification", Brain Res 23 (3): 343–352, DOI:10.1016/0006-8993(70)90061-2
- ↑ Iwaniuk, A.N.; Hurd, P.L.; Wylie, D.R. (2006), "Comparative Morphology of the Avian Cerebellum: I. Degree of Foliation", Brain Behav Evol 68 (1): 45–62, DOI:10.1159/000093530
- ↑ Farris, S.M. & N.S. Roberts (2005), "Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects", Proceedings of the National Academy of Sciences 102 (48): 17394–17399, DOI:10.1073/pnas.0508430102 [e]
- ↑ Prothero, J.W. (1984), "Folding of the Cerebral Cortex in Mammals", Brain Behavior and Evolution 24: 152-167, DOI:10.1159/000121313
- ↑ Mayhew, T.M.; Mwamengele, G.L.; Dantzer, V.; Williams, S. (1996). "The gyrification of mammalian cerebral cortex: quantitative evidence of anisomorphic surface expansion during phylogenetic and ontogenetic development". Journal of Anatomy 188 (Pt 1): 53-58.
- ↑ Marino, L.; R.C. Connor & R. Ewan Fordyce et al. (2007), "Cetaceans Have Complex Brains for Complex Cognition", PLoS Biology 5 (5): e139, DOI:10.1371/journal.pbio.0050139 [e]
- ↑ Hofman, M.A. (1989). "On the evolution and geometry of the brain in mammals.". Prog Neurobiol 32 (2): 137-58. DOI:10.1016/0301-0082(89)90013-0. Research Blogging. [e]
- ↑ Ferrer, I.; I. Fabregues & E. Condom (1986), "A Golgi study of the sixth layer of the cerebral cortex. I. The lissencephalic brain of Rodentia, Lagomorpha, Insectivora and Chiroptera", J Anat 145: 217–234
- ↑ Pillay, P. & P.R. Manger (2007), "Order-specific quantitative patterns of cortical gyrification", European Journal of Neuroscience 25 (9): 2705–2712, DOI:10.1111/j.1460-9568.2007.05524.x
- ↑ Hassiotis, M.; G. Paxinos & K.W.S. Ashwell (2003), "The anatomy of the cerebral cortex of the echidna (Tachyglossus aculeatus)", Comparative Biochemistry and Physiology, Part A 136 (4): 827–850, DOI:10.1016/S1095-6433(03)00166-1 [e]
- ↑ Ferrer, I.; I. Fabregues & E. Condom (1986), "A Golgi study of the sixth layer of the cerebral cortex. II. The gyrencephalic brain of Carnivora, Artiodactyla and Primates", J Anat 146: 87–104
- ↑ Hof, P.R.; R. Chanis & L. Marino (2005), "Cortical Complexity in Cetacean Brains", Anatomical Record Part a Discoveries in Molecular Cellular and Evolutionary Biology 287 (1): 1142, DOI:10.1002/ar.a.20258
- ↑ Hakeem, A.Y.; P.R. Hof & C.C. Sherwood et al. (2005), "Brain of the African elephant (Loxodonta africana): neuroanatomy from magnetic resonance images", Anat Rec A: Discov Mol Cell Evol Biol 287 (1): 1117–1127
- ↑ Reep, R.L. & T.J. O'Shea (1990), "Regional brain morphometry and lissencephaly in the Sirenia", Brain Behav Evol 35 (4): 185–194, DOI:10.1159/000115866
- ↑ Stangier, H. (1937), "Die Furchen der Großhirnrinde beim Schimpanse", Zeitschrift für Anatomie und Entwicklungsgeschichte 107: 647, DOI:10.1007/BF02118571
- ↑ Supèr, H.; Uylings, H.B.M. (2001), "The Early Differentiation of the Neocortex: a Hypothesis on Neocortical Evolution", Cerebral Cortex 11 (12): 1101–1109, DOI:10.1093/cercor/11.12.1101
- ↑ Sereno, M.I. & R.B. Tootell (2005), "From monkeys to humans: what do we now know about brain homologies?", Curr Opin Neurobiol 15 (2): 135–44, DOI:10.1016/j.conb.2005.03.014
- ↑ Chi, J.G.; E.C. Dooling & F.H. Gilles (1977), "Gyral development of the human brain", Annals of Neurology 1 (1): 86–93, DOI:10.1002/ana.410010109
- ↑ Garel, C.; E. Chantrel & M. Elmaleh et al. (2003), "Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination", Child's Nervous System 19 (7): 422–425, DOI:10.1007/s00381-003-0767-4
- ↑ Toi, A.; W.S. Lister & K.W. Fong (2004), "How early are fetal cerebral sulci visible at prenatal ultrasound and what is the normal pattern of early fetal sulcal development?", Ultrasound in Obstetrics and Gynecology 24 (7): 706–715, DOI:10.1002/uog.1802 [e]
- ↑ 25.0 25.1 Regis, J.; J.F. Mangin & T. Ochiai et al. (2005), ""Sulcal root" generic model: a hypothesis to overcome the variability of the human cortex folding patterns", Neurol Med Chir (Tokyo) 45 (1): 1–17, DOI:10.2176/nmc.45.1
- ↑ Ghai, Sandeep; Katherine W. Fong & Ants Toi et al. (2006), "Prenatal US and MR Imaging Findings of Lissencephaly: Review of Fetal Cerebral Sulcal Development", RadioGraphics 26 (2): 389–405, DOI:10.1148/rg.262055059
- ↑ 27.0 27.1 Smart, I.H. & G.M. McSherry (1986), "Gyrus formation in the cerebral cortex in the ferret. I. Description of the external changes", Journal of Anatomy 146: 141-152
- ↑ Neal, J.; M. Takahashi & M. Silva et al. (2007), "Insights into the gyrification of developing ferret brain by magnetic resonance imaging", J Anat 210 (1): 66–77, DOI:10.1111/j.1469-7580.2006.00674.x
- ↑ Robin Highley, J.; L.E. Delisi & N. Roberts et al. (2003), "Sex-dependent effects of schizophrenia: an MRI study of gyral folding, and cortical and white matter", Psychiatry Research: Neuroimaging 124 (1): 11–23, DOI:10.1016/S0925-4927(03)00076-3
- ↑ 30.0 30.1 Zilles, K.; R. Kawashima & A. Dabringhaus et al. (2001), "Hemispheric Shape of European and Japanese Brains: 3-D MRI Analysis of Intersubject Variability, Ethnical, and Gender Differences", Neuroimage 13 (2): 262–271, DOI:10.1006/nimg.2000.0688
- ↑ Magnotta, Vincent A.; Nancy C. Andreasen & Susan K. Schultz et al. (1999), "Quantitative in Vivo Measurement of Gyrification in the Human Brain: Changes Associated with Aging", Cerebral Cortex 9 (2): 151–160, DOI:10.1093/cercor/9.2.151 [e]
- ↑ Bartley, A.J.; Jones, D.W.; Weinberger, D.R. (1997). "Genetic variability of human brain size and cortical gyral patterns". Brain 120 (2): 257-269.
- ↑ Rubenstein, John L.R.; Stewart Anderson & Limin Shi et al. (1999), "Genetic Control of Cortical Regionalization and Connectivity", Cerebral Cortex 9 (6): 524–532, DOI:10.1093/cercor/9.6.524
- ↑ Rubenstein, John L.R. & Pasko Rakic (1999), "Genetic Control of Cortical Development", Cerebral Cortex 9 (6): 521–523, DOI:10.1093/cercor/9.6.521
- ↑ Chenn, Anjen; Walsh, Christopher A. (2002), "Regulation of Cerebral Cortical Size by Control of Cell Cycle Exit in Neural Precursors", Science 297 (5580): 365–9, DOI:10.1126/science.1074192 [e]
- ↑ Kippenhan, J. Shane; Rosanna K. Olsen & Carolyn B. Mervis et al. (2005), "Genetic Contributions to Human Gyrification: Sulcal Morphometry in Williams Syndrome", Journal of Neuroscience 25 (34): 7840, DOI:10.1523/JNEUROSCI.1722-05.2005
- ↑ Kerjan, G. & J.G. Gleeson (2007), "Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly", Trends in Genetics 23 (12): 623–630, DOI:10.1016/j.tig.2007.09.003 [e]
- ↑ Van Essen, D.C. (1997). "A tension-based theory of morphogenesis and compact wiring in the central nervous system". Nature 385 (6614): 313-8.
- ↑ Mangin, J.F.; D. Rivière & A. Cachia et al. (2004), "A framework to study the cortical folding patterns", Neuroimage 23: 129–138, DOI:10.1016/j.neuroimage.2004.07.019
- ↑ Hilgetag, C.C. & H. Barbas (2005), "Developmental mechanics of the primate cerebral cortex", Anat Embryol (Berl) 210 (5-6): 411–7, DOI:10.1007/s00429-005-0041-5
- ↑ Mora, T.; Boudaoud, A. (2006). "Buckling of swelling gels". The European Physical Journal E - Soft Matter 20 (2): 119-124.
- ↑ Hilgetag, C.C. & H. Barbas (2006), "Role of Mechanical Factors in the Morphology of the Primate Cerebral Cortex", PLoS Comput Biol 2 (3): e22, DOI:10.1371/journal.pcbi.0020022
- ↑ Kornack, David R. & Pasko Rakic (1998), "Changes in cell-cycle kinetics during the development and evolution of primate neocortex", Proceedings of the National Academy of Sciences of the United States of America 95 (3): 1242–1246, DOI:10.1073/pnas.95.3.1242 [e]
- ↑ Dehay, C.; P. Giroud & M. Berland et al. (1996), "Contribution of thalamic input to the specification of cytoarchitectonic cortical fields in the primate: Effects of bilateral enucleation in the fetal monkey on the boundaries, dimensions, and gyrification of striate and extrastriate cortex", The Journal of Comparative Neurology 367 (1): 70–89
- ↑ Crino, P.B. & J. Eberwine (1997), "Cellular and Molecular Basis of Cerebral Dysgenesis", Journal of Neuroscience Research 50: 907–916, DOI:<907::AID-JNR1>3.0.CO;2-H 10.1002/(SICI)1097-4547(19971215)50:6<907::AID-JNR1>3.0.CO;2-H
- ↑ Cardoso, C.; R.J. Leventer & J.J. Dowling et al. (2002), "Clinical and Molecular Basis of Classical Lissencephaly: Mutations in the LIS1 Gene (PAFAH1B1)", Human Mutation 19 (1): 4–15, DOI:10.1002/humu.10028
- ↑ Kato, Mitsuhiro & William B. Dobyns (2003), "Lissencephaly and the molecular basis of neuronal migration", Human Molecular Genetics 12 (Review Issue 1): 89–96, DOI:10.1093/hmg/ddg086 [e]
- ↑ Price, D.J. (2004). "Lipids make smooth brains gyrate". Trends in Neurosciences 27 (7): 362-364.
- ↑ Xu, G.; P.V. Bayly & L.A. Taber (2008), "Residual stress in the adult mouse brain", Biomech Model Mechanobiol, DOI:10.1007/s10237-008-0131-4
- ↑ Toro, R.; Perron, M.; Pike, B.; Richer, L.; Veillette, S.; Pausova, Z.; Paus, T. (2008). "Brain Size and Folding of the Human Cerebral Cortex". Cerebral Cortex.
- ↑ Ferrer, I.; I. Fabregues & E. Condom (1987), "A Golgi study of the sixth layer of the cerebral cortex. III. Neuronal changes during normal and abnormal cortical folding", J Anat 152: 71–82
- ↑ Wen, Q. & D.B. Chklovskii (2005), "Segregation of the Brain into Gray and White Matter: A Design Minimizing Conduction Delays", PLoS Comput Biol 1 (7): e78, DOI:10.1371/journal.pcbi.0010078
- ↑ Dubois, J.; M. Benders & C. Borradori-tolsa et al. (2008), "Primary cortical folding in the human newborn: an early marker of later functional development", Brain 131 (8): 2028, DOI:10.1093/brain/awn137 [e]
- ↑ Lüders, Eileen; Narr, Katherine L.; Bilder, Robert M.; Szeszko, Philip R.; Gurbani, Mala N.; Hamilton, Liberty; Toga, Arthur W.; Gaser, Christian (2007), "Mapping the Relationship between Cortical Convolution and Intelligence: Effects of Gender", Cerebral Cortex 18 (9): 2019, DOI:10.1093/cercor/bhm227
- ↑ Lüders, E.; K.L. Narr & P.M. Thompson et al. (2004), "Gender differences in cortical complexity", Nat Neurosci 7 (8): 799–800, DOI:10.1038/nn1277
- ↑ Barkovich, A.J.; T.K. Koch & C.L. Carrol (1991), "The spectrum of lissencephaly: report of ten patients analyzed by magnetic resonance imaging", Ann Neurol 30 (2): 139–46, DOI:10.1002/ana.410300204
- ↑ Liang, J.S.; W.T. Lee & C. Young et al. (2002), "Agyria-pachygyria: Clinical, neuroimaging, and neurophysiologic correlations", Pediatric neurology 27 (3): 171–176, DOI:10.1016/S0887-8994(02)00401-0 [e]
- ↑ Ramirez, D.; E.J. Lammer & C.B. Johnson et al. (2004), "Autosomal recessive frontotemporal pachygyria", American Journal of Medical Genetics 124 (3): 231–238, DOI:10.1002/ajmg.a.20388 [e]
- ↑ Kurul, S.; H. Çakmakçi & E. Dirik (2004), "Agyria-pachygyria complex: MR findings and correlation with clinical features", Pediatric Neurology 30 (1): 16–23, DOI:10.1016/S0887-8994(03)00312-6 [e]
- ↑ Hager, B.C.; I.Z. Dyme & S.R. Guertin et al. (1991), "Linear nevus sebaceous syndrome: megalencephaly and heterotopic gray matter", Pediatr Neurol 7 (1): 45–9, DOI:10.1016/0887-8994(91)90105-T
- ↑ White, T.; Andreasen, N.C.; Nopoulos, P.; Magnotta, V. (2003), "Gyrification abnormalities in childhood- and adolescent-onset schizophrenia", Biological Psychiatry 54 (4): 418–426, DOI:10.1016/S0006-3223(03)00065-9
- ↑ Cachia, A.; M.L. Paillère-Martinot & A. Galinowski et al. (2007), "Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations", Neuroimage
- ↑ Hardan, A.Y.; R.J. Jou & M.S. Keshavan et al. (2004), "Increased frontal cortical folding in autism: a preliminary MRI study", Psychiatry Research: Neuroimaging 131 (3): 263–268, DOI:10.1016/j.pscychresns.2004.06.001
- ↑ Ronan, L.; K. Murphy & N. Delanty et al. (2007), "Cerebral Cortical Gyrification: A Preliminary Investigation in Temporal Lobe Epilepsy", Epilepsia 48 (2): 211–219, DOI:10.1111/j.1528-1167.2006.00928.x
- ↑ Casanova, Manuel F.; Julio Araque & Jay Giedd et al. (2004), "Reduced Brain Size and Gyrification in the Brains of Dyslexic Patients", Journal of Child Neurology 19 (4): 275-281, DOI:10.1177/088307380401900407 [e]
- ↑ Bearden, Carrie E.; Theo G.M. Van Erp & Rebecca A. Dutton et al. (2009), "Alterations in Midline Cortical Thickness and Gyrification Patterns Mapped in Children with 22q11.2 Deletions", Cerebral Cortex 19 (1): 115, DOI:10.1093/cercor/bhn064
- ↑ Wolosin, Sasha M. (2009), "Abnormal cerebral cortex structure in children with ADHD", Human Brain Mapping 30: 175-184, DOI:10.1002/hbm.20496
- ↑ Schmitt, J.E.; Watts, K.; Eliez, S.; Bellugi, U.; Galaburda, A.M.; Reiss, A.L. (2002). "Increased gyrification in Williams syndrome: evidence using 3D MRI methods". Developmental Medicine & Child Neurology 44 (5): 292-295. DOI:10.1111/j.1469-8749.2002.tb00813.x. Research Blogging.
- ↑ Vogeley, K.; T. Schneider-Axmann & U. Pfeiffer et al. (2000), "Disturbed Gyrification of the Prefrontal Region in Male Schizophrenic Patients: A Morphometric Postmortem Study", American Journal of Psychiatry 157 (1): 34-39
- ↑ Kulynych, J.J.; L.F. Luevano & D.W. Jones et al. (1997), "Cortical abnormality in schizophrenia: an in vivo application of the gyrification index", Biological Psychiatry 41 (10): 995–999, DOI:10.1016/S0006-3223(96)00292-2
- ↑ Sallet, Paulo C.; Helio Elkis & Tania M. Alves et al. (2003), "Reduced Cortical Folding in Schizophrenia: an MRI Morphometric Study", American Journal of Psychiatry 160 (9): 1606-1613, DOI:10.1176/appi.ajp.160.9.1606 [e]
- ↑ Rodriguez-Carranza, C.E.; P. Mukherjee & D. Vigneron et al. (2008), "A framework for in vivo quantification of regional brain folding in premature neonates", Neuroimage 41: 462, DOI:10.1016/j.neuroimage.2008.01.008
- ↑ Pienaar, R.; B. Fischl & V. Caviness et al. (2008), "A methodology for analyzing curvature in the developing brain from preterm to adult", International Journal of Imaging Systems and Technology 18 (1): 42–68, DOI:10.1002/ima.20138
- CZ Live
- Biology Workgroup
- Computers Workgroup
- Health Sciences Workgroup
- Differential geometry Subgroup
- Image analysis Subgroup
- Neuroscience Subgroup
- Articles written in British English
- All Content
- Biology Content
- Computers Content
- Health Sciences Content
- Differential geometry tag
- Image analysis tag
- Neuroscience tag