Nuclear chemistry: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng |
||
| Line 23: | Line 23: | ||
Another key area within radiation chemistry is the modification of polymers,[http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=7313004][http://mitr.p.lodz.pl/biomat/raport/3_5_radiation_hydrogels.html] it is possible to convert [[monomer]]s to [[polymer]]s with radiation, to crosslink polymers and to break polymer chains using irradation. Both man-made and natural polymers (such as [[carbohydrate]]s)[http://www-pub.iaea.org/MTCD/publications/PDF/te_1422_web.pdf] can be processed using these methods. | Another key area within radiation chemistry is the modification of polymers,[http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=7313004][http://mitr.p.lodz.pl/biomat/raport/3_5_radiation_hydrogels.html] it is possible to convert [[monomer]]s to [[polymer]]s with radiation, to crosslink polymers and to break polymer chains using irradation. Both man-made and natural polymers (such as [[carbohydrate]]s)[http://www-pub.iaea.org/MTCD/publications/PDF/te_1422_web.pdf] can be processed using these methods. | ||
To process materials, either a gamma source or an electron beam can be used. The internation type IV (''wet storage'') irradiator is a common design the JS6300 and JS6500 gamma sterilizers | To process materials, either a gamma source or an electron beam can be used. The internation type IV (''wet storage'') irradiator is a common design the JS6300 and JS6500 gamma sterilizers <ref>made by 'Nordion International', which used to trade as ,Atomic Energy of Canada Ltd,') are typical designs. <ref>Many features of the design are discussed in the [[IAEA]] report on a [[human error]] accident involving such a irradiation plant [http://www-pub.iaea.org/MTCD/publications/PDF/Pub847_web.pdf]</ref>. In these irradiation plants, the source is stored in a deep well filled with water when not in use. When the source is required, it is moved by a steel wire to the irradiation room where the products which are to be treated are present; these objects are placed inside boxes which are moved through the room by an automatic mechanism. By moving the boxes from one point to another in the room, the contents are given a uniform dose. After treatment, the product is moved by the automatic mechanism out of the irradiation room. The irradiation room has very thick concrete walls (circa 3 m) to prevent gamma rays from escaping. The source is <sup>60</sup>Co rods which are sealed within two layers of stainless steel, the rods are combined with inert dummy rods to form a rack which has a total activity of about 12.6 PBq (340 kCi). | ||
===Study of nuclear reactions=== | ===Study of nuclear reactions=== | ||
Revision as of 11:07, 30 December 2006
Nuclear chemistry is a subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties.
- It includes the chemistry of radioactive elements such as the actinides, radium and radon together with the chemistry associated with equipment (such as nuclear reactors) which are designed to perform nuclear processes. This includes the corrosion of surfaces and the behaviour under conditions of both normal and abnormal operation (such as during an accident). An important area is the behaviour of objects and materials after being placed into a waste store or otherwise disposed of.
- Nuclear chemistry also includes the study of the production and use of radioactive sources for a range of processes which include radiotherapy; The use of tracers within industry, science and the environment; The use of radiation to modify materials such as polymers[5][6] and polychlorobiphenyls.
- Nuclear chemistry also involves the study and use of nuclear processes in non-radioactive areas of human activity. For instance, nuclear magnetic resonance (NMR) spectroscopy is commonly used in synthetic organic chemistry.
Early history
After the discovery of X-rays by Wilhelm Röntgen, many scientists began to work on ionizing radiation. One of these was Henri Becquerel, who investigated the relationship between phosphorescence and the blackening of photographic plates. When Becquerel (working in France) discovered that, with no external source of energy, the uranium generated rays which could blacken (or fog) the photographic plate, radioactivity was discovered. Marie Curie (working in Paris) and her husband Pierre Curie isolated two new radioactive elements from uranium ore. They used radiometric methods to identify which stream the radioactivity was in after each each chemical separation; they separated the uranium ore into each of the different chemical elements that were known at the time, and measured the radioactivity of each fraction. They then attempted to separate these radioactive fractions further, to isolate a smaller fraction with a higher specific activity (radioactivity divided by mass). In this way, they isolated polonium and radium. Their daughter Irène Joliot-Curie and her husband were the first to 'create' radioactivity: they bombarded boron with alpha particles to make a proton-rich isotope of nitrogen; this isotope emitted positrons.[7] In addition, they bombarded aluminium and magnesium with neutrons to make new radioisotopes.
Ernest Rutherford, working in Canada and England, discovered that radioactivity decays according to first order kinetics (half life); he coined the terms alpha, beta and gamma rays. He also converted nitrogen into oxygen, and most importantly he supervised the students who did the Geiger-Marsden experiment (gold leaf experiment) which showed that the 'plum pudding model' of the atom was wrong. In the plum pudding model, proposed by J. J. Thomson in 1904, the atom is composed of electrons surrounded by a 'cloud' of positive charge to balance the electrons' negative charge. To Rutherford, the gold foil experiment implied that the positive charge was confined to a very small nucleus leading first to the Rutherford model, and eventually to the Bohr model of the atom.
Main areas
Radiochemistry
Radiochemistry is the chemistry of radioactive materials. In radiochemistry, radioactive isotopes of elements are used to study the properties and chemical reactions of ordinary non-radioactive isotopes (often within radiochemistry the absence of radioactivity leads to a substance being described as being inactive as the isotopes are stable). An example of a biological use of radiochemistry is the study of DNA using radioactive phosphorus-32.
Radiochemistry also includes the study of the behaviour of radioisotopes within the environment, for instance V.I. Yoschenko et. al. in Journal of Environmental Radioactivity, 2006, 86, 143-163 report how a forest or grass fire can make radioisotopes become mobile again. As an experiment, fires were set within the exclusion zone around chernobyl and the levels of the radioactivity in the air down wind of these fires was measured.
Radiation chemistry
Radiation chemistry is the study of the chemical effects of radiation on matter; this is very different to radiochemistry as no radioactivity needs to be present in the material which is being chemically changed by the radiation. An example is the conversion of water into hydrogen gas and hydrogen peroxide. A recent area of work has been the destruction of toxic organic compounds by irradiation [1]; after irradiation, "dioxins" (polychlorodibenzo-p-dioxins) are dechloroinated in the same way as PCBs can be converted to biphenyl an inorganic chloride. This is due to the reaction of the solvated electron with the organic compound to form a radical anion which decomposes by the loss of a chloride anion. If a deoxygenated mixture of PCBs in isopropanol or mineral oil is irradiated with gamma rays, then the PCBs will be dechlorinated to form inorganic chloride and biphenyl. The reaction works best in isopropanol if potassium hydroxide (caustic potash) is added. Solvated electrons have been shown to be responsible for the reaction. If oxygen, nitrous oxide, sulfur hexafluoride or nitrobenzene is present in the mixture, then the reaction rate is reduced. This work has been done recently in the USA, often with used nuclear fuel as the radiation source[8][9].
Another key area within radiation chemistry is the modification of polymers,[10][11] it is possible to convert monomers to polymers with radiation, to crosslink polymers and to break polymer chains using irradation. Both man-made and natural polymers (such as carbohydrates)[12] can be processed using these methods.
To process materials, either a gamma source or an electron beam can be used. The internation type IV (wet storage) irradiator is a common design the JS6300 and JS6500 gamma sterilizers Cite error: Closing </ref> missing for <ref> tag. In these irradiation plants, the source is stored in a deep well filled with water when not in use. When the source is required, it is moved by a steel wire to the irradiation room where the products which are to be treated are present; these objects are placed inside boxes which are moved through the room by an automatic mechanism. By moving the boxes from one point to another in the room, the contents are given a uniform dose. After treatment, the product is moved by the automatic mechanism out of the irradiation room. The irradiation room has very thick concrete walls (circa 3 m) to prevent gamma rays from escaping. The source is 60Co rods which are sealed within two layers of stainless steel, the rods are combined with inert dummy rods to form a rack which has a total activity of about 12.6 PBq (340 kCi).
Study of nuclear reactions
see also nuclear physics
A combination of radiochemistry and radiation chemistry is used to study nuclear reactions such as fission and fusion. Some early evidence for nuclear fission was the formation of a shortlived radioisotope of barium which was isolated from neutron irradated uranium ( 139Ba, with a half-life of 83 minutes and 140Ba, with a half-life of 12.8 days, are major fission products of uranium). At the time, it was thought that this was a new radium isotope, as it was then standard radiochemical practise to use a barium sulphate carrier precipitate to assist in the isolation of radium.[13]. More recently, a combination of radiochemical methods and nuclear physics has been used to try to make new 'superheavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated. For more details of the original discovery of nuclear fission see the work of Otto Hahn.[2].
Radioisotope production
The processes forming new isotopes (often radioactive) involve several areas of nuclear chemistry.
Processes
- By irradiation with slow neutrons, it is possible to form neutron-rich isotopes which tends to decay by beta decay (i.e. by electron emission from the nuclei). For instance, irradiating 59Co with neutrons forms an excited state of 60Co (best written as 60mCo) which decays by emitting a gamma ray to the ground state of 60Co, which in turn decays by emitting an electron to form 60mNi. The excited state of the 60mNi then decays with the emission of two gamma photons to the ground state of 60Ni. As the neutron energy increases, the simple capture reactions become less important, while other reactions such as the (n,p) reaction become more important. An example is the production of phosphorus-32 by neutron irradiation of 32S. The sulphur nucleus captures a neutron and emits a proton to form the radioactive phosphorus isotope ( 32P). Carbon-14 is obtained in a similar manner by irradiating 14N with neutrons.
- A beam of fast moving positive particles can be obtained by using a cyclotron or a linear accelerator (linac); up to 30MeV protons and deuterons can be obtained this way. The energies of these particles are so high that they can overcome the electrostatic barrier which opposes the entry of positive particles into the nucleus. An example of the use of the (p,n) reaction is the conversion of 103Rh into 103Pd, this can be done by irradiating rhodium foil with protons to form the radioactive palladium isotope. The reaction of beryllium with alpha particles is another example of this type of reaction. While the reaction of 9Be with 4He2+ generates 12C, its most important aspect is that it generates neutrons.
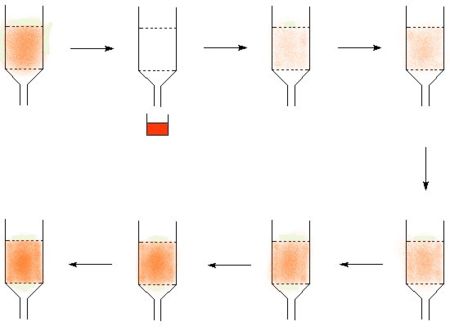
- Many isotopes can be made from a parent isotope which decays to form the desired isotope. If the parent and the product element can be chemically separated, then it is possible to create an "isotope cow". The classic isotope cow is the technetium cow, many others work by the same principle. The technetium cow uses molybdum-99 absorbed on alumina, and it is "milked" by passing saline solution through it to give a solution of technetium.
In the diagram, the technetium is represented in red, in picture two the cow is milked to make a product solution. The technetium then builds up again to allow the cow to return to the technetium loaded state where it can be milked again.
In this way, aqueous solutions of the following isotopes can be made from parent isotopes (shown in brackets)
- 68Ga (68Ge)
- 82Rb (82Sr)
- 99mTc (99Mo)
- 113mIn (113Sn)
- 188Re (188W)
- 62Cu (62Zn)
When the product isotope is a gas, the cow can be milked by allowing the product to diffuse out of a solid. An early way of making radiography sources was to milk radon from a radium source; this method was used by Marie Curie during the first World War (WWI), and was used in the USA to make Brachytherapy sources. By this method, the following isotopes can be obtained from parent isotopes (shown in brackets)
- 81mKr (81Rb)
- 222Rn (226Ra)
In some nuclear materials, new isotopes are formed by the decay of a parent isotope. For instance, the beta decay of 241Pu will form 241Am, so if a sample of plutonium which has been standing for several years is subjected to a new chemical purification, then it is possible to harvest the americium.
- 241Am (241Pu)
Uses
Radioactive sources have many different uses [3]. A sealed source is sealed within a container so that, in normal use, no radioactive material is lost from the source. In many sealed sources, the radioactive filling is surrounded by one or more layers of a corrosion-resistant material (such as stainless steel or gold). Alternatively, it is possible to form a source using material which holds the radioactivity in a chemically resistant and strong form without needing a metal cover. In designing sealed sources, it is common to choose a chemically stable form of the radioactive element, but for cesium radiotherapy sources it is common to use the water soluble chloride, because it is impossible to obtain the required density of cesium in another compound.
- Sealed sources are used for radiotherapy treatment of many cancers (both brachytherapy and teletherapy), as well as for food irradiation, industrial radiography, nuclear gauges and many other applications.
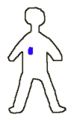
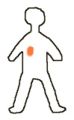
To explain the use of radioactive sources in cancer treatment we will consider a hypothetical person with a tumor (shown in blue).
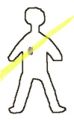
As an alternative the person might be treated with a smaller radioactive source which is placed within or close to the tumor (brachytherapy). Here the source is shown as the red dot while the gamma rays from the source are shown in yellow. One of the advantages of this type of treatment is that the radiation is confined to the part of the person where the disease is, the healthy tissues in other parts of the person are not subjected to irradation.
- Open sources are used for a range of applications which include the use of tracers to study the physical operation of industrial processes, to trace the chemical mechanism by which a product forms. For instance, krypton has been used to study the underground combustion of fuels such as oil and coal.[14][15]. They are also used for some forms of radiotherapy. For example, in the treatment of thyroid cancer the patient is given a large dose of 131I becuase the iodine accumulates in the thyroid gland, the tissue of the thyroid gland (and the tummor) suffers a large radiation dose when compaired with the majority of the body. As a result the radioactive iodine is able to selectively destroy the thyroid gland and the tumor which is derived from it. Also in terminal care 89Sr is used to destroy bone tumors. (See W.A. Volkert and T.J. Hoffman, Chemical Reviews, 1999, 2269-2292 for further details on open source radiotherapy).
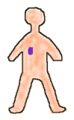
The use of an open source in either cancer treatment or imaging can be explained using the following two diagrams, in the first diagram the person has been recently given the radioactive drug. The drug is spread throughout the whole body. Then with time the radioactivity will concentrate (according to its chemical properties) within a target organ or tumor.
Some radiopharmaceuticals are used for medical imaging, for instance a great array of different technetium complexes are used medically (See S.S. Jurisson and J.D. Lydon, Chemical Reviews, 1999, 2205-2218 for further detail) while radioactive 201Tl (half-life of 73 hours) is used for diagnostic purposes in nuclear medicine, particularly in stress tests used for risk stratification in patients with coronary artery disease (CAD).[4][5] This isotope of thallium can be generated using a transportable generator which is similar to the technetium cow.[6] The generator contains lead-201 (half life 9.33 hours) which decays by electron capture to the 201Tl. The 201Pb can be produced in a cyclotron by the bombardment of thallium with protons or deuterons by the (p,3n) and (d,4n) reactions.[7]
The nuclear fuel cycle
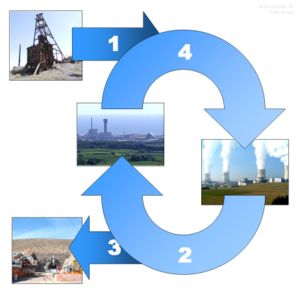
The chemistry associated with any part of the nuclear fuel cycle which includes nuclear reprocessing. The fuel cycle includes all the operations involved in producing fuel, from mining, ore processing, enrichment and fuel production (Front end of the cycle). It also includes the 'in-pile' behaviour (use of the fuel in a reactor) before the back end of the cycle. The back end includes the management of the used nuclear fuel in either a cooling pond or dry storage, before it is either disposed of into an underground waste store or reprocessed.
The study of used fuel
Used nuclear fuel is studied in post irradation examination, where used fuel is examined to know more about the processes that occur in fuel during use, and how these might alter the outcome of an accident. For example, during normal use, the fuel expands due to thermal expansion. This causes cracking, and in extreme cases, such as during the power surge which destroyed the Chernobyl nuclear reactor in April, 1986, the fuel can shatter into very small fragments. Most nuclear fuel is uranium dioxide, which is a cubic solid which has a structure similar to that of calcium fluoride, in used fuel the solid state structure of most of the solid remains the same as that of pure cubic uranium dioxide. SIMFUEL is the name given to the simulated spent fuel which is made by mixing finely ground metal oxides, grinding as a slurry, spray drying it before heating in hydrogen/argon to 1700 oC. [8] In SIMFUEL, 4.1% of the volume of the solid was in the form of metal nanoparticles which are made of molybdenum, ruthenium, rhodium and palladium. Most of these metal particles are of the ε phase (hexagonal) of Mo-Ru-Rh-Pd alloy, while smaller amounts of the α (cubic) and σ (tetragonal) phases of these metals were found in the SIMFUEL. Also present within the SIMFUEL was a cubic perovskite phase which is a barium strontium zirconate (BaxSr1-xZrO3).
Uranium dioxide is very insoluble in water, but after oxidation it can be converted to uranium trioxide or another uranium(VI) compound which is much more soluble in water. It is important to understand that uranium dioxide (UO2) can be oxidised to an oxygen rich hyperstoichiometric oxide (UO2+x) which can be further oxidised to U4O9, U3O7, U3O8 and UO3.2H2O.
Because used fuel contains alpha emitters (plutonium and the minor actinides), the effect of adding an alpha emitter (238Pu) to uranium dioxide on the leaching rate of the oxide has been investigated. For the crushed oxide, adding 238Pu did tend to increase the rate of leaching, but the difference in the leaching rate between between 0.1 and 10% 238Pu was very small. [9]
The concentration of carbonate in the water which is in contact with the used fuel has a considerable effect on the rate of corrosion, because uranium(VI) forms soluble anionic carbonate complexes such as [UO2(CO3)2]2- and [UO2(CO3)3]4-. When carbonate ions are absent, and the water is not strongly acidic, the hexavalent uranium compounds which form on oxidation of uranium dioxide often form insoluble hydrated uranium trioxide phases. [10].
By ‘sputtering’, using uranium metal and an argon/oxygen gas mixture, thin films of uranium dioxide can be deposited upon gold surfaces. These gold surfaces which are modified with uranium dioxide have been used for both cyclic voltammetry and AC impedance experiments, and these offer an insight into the likely leaching behaviour of uranium dioxide. [11]
Fuel cladding interactions
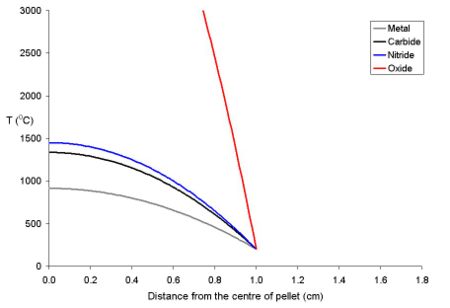
The study of the nuclear fuel cycle includes both the study of the behaviour of nuclear materials both under normal conditions and under accident conditions. For example, there has been much work on how uranium dioxide based fuel interacts with the zirconium alloy tubing used to cover it. During use, the fuel swells due to thermal expansion and then starts to react with the surface of the zirconium alloy, forming a new layer which contains both fuel and zirconium (from the cladding). Then, on the fuel side of this mixed layer, there is a layer of fuel which has a higher cesium to uranium ratio than most of the fuel. This is because xenon isotopes are formed as fission products that diffuse out of the lattice of the fuel into voids such as the narrow gap between the fuel and the cladding. After diffusing into these voids it decays to cesium isotopes. Because of the thermal gradient which exists in the fuel during use, the volatile fission products tend to be driven from the centre of the pellet to the rim area.[12]. Below is a graph of the temperature of uranium metal, uranium nitride and uranium dioxide as a function of the distance from the centre of a 20 mm diameter pellet with a rim temperature of 200 oC. It is important to note that the uranium dioxide (because of its poor thermal conductivity) will overheat at the centre of the pellet, while the more thermally conductive other forms of uranium remain below their melting points.
Absorption of fission products on surfaces
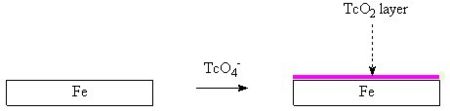
Another important area of nuclear chemistry is the study of the interaction of fission products with surfaces. This is thought to control the rate of release and migration of fission products both from waste containers under normal conditions and from power reactors under accident conditions. It is interesting to note that, like chromate and molybdate, the 99TcO4 anion can react with steel surfaces to form a corrosion resistant layer. In this way, these metaloxo anions act as anodic corrosion inhibitors. The formation of 99TcO2 on steel surfaces is one effect which will retard the release of 99Tc from nuclear waste drums and nuclear equipment which has been lost before decontamination (eg submarine reactors lost at sea). This 99TcO2 layer renders the steel surface passive, it inhibits the anodic corrosion reaction. It has also been shown that 99TcO4 anions react to form a layer on the surface of activated carbon (charcoal).
In a similar way, the release of iodine-131 in a serious power reactor accident could be retarded by absorption on metal surfaces within the nuclear plant. [13]
Spinout areas
Some methods first developed within nuclear chemistry and physics have become so widely used within chemistry and other physical sciences that they may be best thought of as not being part of normal nuclear chemistry. For example, Isotopic chemistry could be included in this subtopic, but due to the use of the isotope effect to investigate chemical mechanisms and the use of cosmogenic isotopes and long-lived unstable isotopes in geology it is best to consider much of isotopic chemistry as separate from nuclear chemistry.
Kinetics (use within mechanistic chemistry)
The mechanisms of chemical reactions can be investigated by observing the effect upon the kinetics of making an isotopic modification of a substrate in a reaction. This is now a standard method in organic chemistry. Briefly, replacing normal hydrogens (protons) within a chemical compound with deuterium causes the rate of molecular vibration (C-H, N-H and O-H bonds show this) to decrease, this then can lead to a decrease in the reaction rate if the rate-determining step involves breaking a bond between hydrogen and another atom. Thus, if the reaction changes in rate when protons are replaced by deuteriums, it is reasonable to assume that the breaking of the bond to hydrogen is part of the step which determines the rate.
Uses within geology, biology and forensic science
Cosmogenic isotopes are formed by the interaction of cosmic rays with the nucleus of an atom. These can be used for dating purposes and for use as natural tracers. In addition, by careful measurement of some ratios of stable isotopes it is possible to obtain new insights into the origin of bullets, ages of ice samples, ages of rocks, and the diet of a person can be identified from a hair or other tissue sample. (See Isotope geochemistry and Isotopic signature for further details).
Nuclear magnetic resonance (NMR)
NMR spectroscopy uses the net spin of nuclei in a substances upon energy absorption, and is used to identify molecules. This has now become a standard spectrscopic tool within synthetic chemistry One of the main uses of NMR is to determine the bond connectivity within an organic molecule.
NMR imaging also uses the net spin of nuclei (commonly protons) for imaging. This is widely used in medicine, and can provide detailed images of the inside of a person without inflicting any radiation upon them. In a medical setting, NMR is often known simply as "Magnetic resonance" imaging, as the word 'nuclear' has negative connotations for many people.
References
- ↑ Zhao C et al (2007) Radiation Physics and Chemistry, 76:37-45
- ↑ Meitner L, Frisch OR (1939) Disintegration of uranium by neutrons: a new type of nuclear reaction Nature 143:239-240 [1]
- ↑ A short review of the use of radioactivity in industry
- ↑ Thallium Test from Walter Reed Army Medical Center
- ↑ Thallium Stress Test from the American Heart Association
- ↑ Abstract
- ↑ Thallium-201 production from Harvard Medical School's Joint Program in Nuclear Medicine
- ↑ A good report on the microstructure of used fuel is Lucuta PG et al (1991) J Nuclear Materials 178:48-60
- ↑ V.V. Rondinella VV et al (2000) Radiochimica Acta 88:527-531
- ↑ for a review of the corrosion of uranium dioxide in a waste store which explains much of the chemistry, see Shoesmith DW (2000) J Nuclear Materials 282:1-31
- ↑ Miserque F et al (2001) J Nuclear Materials 298:280-90
- ↑ Further reading on fuel cladding interactions: Tanaka K et al (2006) J Nuclear Materials 357:58-68
- ↑ * Glänneskog H (2004) Interactions of I2 and CH3I with reactive metals under BWR severe-accident conditions Nuclear Engineering and Design 227:323-9
Text books
- Radiochemistry and Nuclear Chemistry
Comprehensive textbook by Choppin, Liljenenzin and Rydberg. ISBN -0750674636, Butterworth-Heinemann, 2002.
- Radioactivity, Ionizing radiation and Nuclear Energy
Basic textbook for undergraduates by Hála and Navratil. ISBN -807302053-X, Konvoj, 2003
- The Radiochemical Manual
Overview of the production and uses of both open and sealed sources. Edited by BJ Wilson and written by RJ Bayly, JR Catch, JC Charlton, CC Evans, TT Gorsuch, JC Maynard, LC Myerscough, GR Newbery, H Sheard, CBG Taylor and BJ Wilson. The radiochemical centre (Amersham) was sold via HMSO, 1966 (second edition)