imported>Gareth Leng |
|
| (28 intermediate revisions by one other user not shown) |
| Line 1: |
Line 1: |
| {{CZ:(U00984) Appetite and Obesity, University of Edinburgh 2010/EZnotice}}
| |
| {{subpages}} | | {{subpages}} |
| The regulation of appetite involves a complex interplay between circulating hormones, neurotransmitters, neuropeptides and nutrients, and the melanocortin system is a central component in this. | | '''Melanocortins''' are peptides derived from [[pro-opiomelanocortin]] (POMC), determined by tissue-specific post-translational cleavage. The regulation of [[appetite]] involves a complex interplay between circulating hormones, neurotransmitters, neuropeptides and nutrients, and the melanocortin system is an important component of this. <ref>Cone R (2006) Studies on the Physiological functions of the melanocortin system ''Endocrine reviews'' [http://edrv.endojournals.org/cgi/content/full/27/7/736 27:736-49]</ref><ref>Qian G, Tamas H (2007) Neurobiology of feeding and energy expenditure ''Annu Rev Neurosci'' [http://www.annualreviews.org/doi/full/10.1146/annurev.neuro.30.051606.094324 30:367-98]</ref><ref>Seeley R ''et al.'' (2004) The critical role of The melanocortin system in the control of energy balance ''Annu Rev Nutrition'' [http://www.annualreviews.org/doi/full/10.1146/annurev.nutr.24.012003.132428?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed 24:133-49]</ref><ref>Mountjoy K (2010) Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes ''Biochem J'' [http://www.biochemj.org/bj/428/0305/4280305.pdf 428:305-24]</ref><ref>Fan W ''et al.'' (2000) The central melanocortin system can directly regulate serum insulin levels ''Endocrinology'' 141:3072-9</ref><ref>Gantz I, Fong TM (2003) The melancortin system ''Am J Physiol'' 284:E468-74</ref> |
|
| |
|
| [[Pro-opiomelanocortin]] (POMC) is a pro-hormone that gives rise to an array of bioactive peptide hormones that are implicated in energy balance, stress responses, pain, immune modulation, satiety, [[pigmentation]] and even [[exocrine gland]] secretions. It is expressed in the [[pituitary gland]], skin, [[immune system]] and brain. In the brain, it is expressed in the [[arcuate nucleus]] of the [[hypothalamus]] and the [[nucleus tractus solitarii]] (NTS) of the caudal brainstem. POMC is post-translationally cleaved by a complex set of enzymes into a variety of hormones.
| | {{Image|MC4R PATHWAYS.JPG|centre|650px|Circulating molecules signal to the arcuate nucleus the nutritional state of the body}} |
|
| |
|
| {{Image|POMC.JPG|centre|700px|Fig1: Simplified diagram of the breakdown of POMC by prohormone convertase enzymes into the melanocortins and opioid peptide B endorphin}}
| | POMC is expressed in many tissues including the [[pituitary gland]] and the [[brain]], and its products are involved in many different physiological processes. In the brain, POMC is expressed in the [[arcuate nucleus]] of the [[hypothalamus]] and the [[nucleus tractus solitarii]] (NTS) of the caudal brainstem, and products of POMC are important in appetite regulation. POMC was first noted for its importance in appetite and obesity in rodent studies. POMC-null mice exhibit [[hyperphagia]] and [[obesity]]. The suggestion that POMC has a role in [[appetite]] and energy balance is supported by studies on rare individuals with POMC mutations. In humans, a lack of POMC is generally fatal unless glucocorticoids are administered from birth as [[cortisol]] is essential for humans, but a few rare individuals with the mutation have shown very similar phenotype to the POMC knockout mouse. <ref>Oswal A, Yeo GSH (2007) The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics ''Obesity Rev'' 8:293–306</ref><ref>Farooqi IS, O’Rahilly S(2008) Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. nature clinical practice ''Endocrinol Metabol'' </ref> |
|
| |
|
| Both the [[opioid]] peptide [[B endorphin]] and the melanocortin [[α-melanocyte stimulating hormone]] (α-MSH) are cleaved from POMC; α-MSH is known to reduce feeding behaviour, but β endorphin increases feeding behaviour (especially of highly palatable foods). It is therefore important to explore how the prohormone may be processed in different tissues and what dictates this. In the same sense, how are the cleavage enzymes regulated to produce various concentrations of the different peptide hormones? | | POMC expression in the arcuate nucleus is regulated by [[leptin]], a hormone secreted by adipose tissue. Both the [[opioid]] peptide [[B endorphin]] and the melanocortin [[alpha-melanocyte stimulating hormone]] (α-MSH) are cleaved from POMC; α-MSH is a very potent inhibitor of feeding behaviour, but β endorphin increases feeding behaviour (especially of highly palatable foods). It is therefore important to explore how the prohormone may be processed in different tissues and what dictates this. In the same sense, how are the cleavage enzymes regulated to produce various concentrations of the different peptide hormones? In the brain, the actions of α-MSH are mediated via specific melanocortin receptors - particulary MC3 and MC4 receptors. These receptors are unusual in that they have both endogenous agonists and antagonists. |
|
| |
|
| Experiments in which the [[propeptide convertase]] (PC) enzymes are knocked out are not particularly helpful as PC1 and PC2 are expressed in cells other than POMC cells and have important physiological functions other than POMC cleavage. For example PC1 is essential for the biosynthesis of [[insulin]] and PC2 for the biosynthesis of [[glucagon]]. However, transgenic mice deficient in PC2 (PC2 'knockout' mice) exhibit no α-MSH expression at all, and [[Prader Willi]] patients have reduced hypothalamic PC2 expression. <ref>Millington ''et al.'' (2003)</ref> The PC2 knockout mice are so defective in other ways that it is hard to tell whether the lack of alpha MSH has an effect.<ref>Scamuffa ''et al.'' (2006)</ref> Prader Willi sufferers do exhibit an obese phenotype but again this syndrome comes as a result of a mutation of several genes so it cannot be inferred that PC2 mutation is solely responsible for the obese and hyperphagic phenotype.
| | ==Agouti and agouti-related peptide== |
| | The central melanocortin system involves several agonists such as α, β, γ MSH and two inverse agonists, [[agouti]] and AgRP. These act on five subtypes of MC receptors (MCR1-5). <ref>Wikberga, J ''et al.'' (2000) New Aspects on the melanocortins and their receptors ''Pharmacol Res'' 42: 393-420</ref> |
| | Of these, AgRP and α-MSH are thought to be most important for appetite regulation mainly via their actions on MC4 receptors. In addition to suppressing appetite, α-MSH increases both metabolism and body temperature. <ref>Balasko ''et al.'' (2010) Central alpha-MSH, energy balance, thermal balance and antipyresis ''J Thermal Biol'' 35:211-7 |
| | </ref>Some MC4 receptors have been found on adipocytes, suggesting that circulating melanocortins may also be involved in regulating energy homeostasis. |
|
| |
|
| Some evidence that leptin modifies POMC processing as leptin-deficient mice show an upregulation of POMC and PC2 expression. <ref>Pritchard ''et al'' 2002)</ref> You cannot however infer from this that the proteins themselves are increased nor does it mean that α-MSH production is increased.
| | Agouti is an antagonist at MC1 and MC4 receptors, while AgRP iis an inverse agonist at MC3 and MC4 receptors. Mutant mice with ectopic expression of these peptides are hyperphagic with an increase in adipose mass, lean mass, and hyperinsulinemia. <ref>Pritchard L ''et al.''(2002) Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity ''J Endocrinol'' [http://joe.endocrinology-journals.org/cgi/reprint/172/3/411 172:411-21]</ref> |
|
| |
|
| Some recent experiments have shown that, contrary to previous belief, B-endorphin may act in a complementary manner with α-MSH in the long term as BEND-/- male mice are obese, suggesting that β-endorphin has an unexpected anorexic phenotype <ref>Appleyard et al 2003)</ref>
| |
|
| |
|
| | {{Image|POMC.JPG|centre|700px|The breakdown of POMC by prohormone convertase enzymes (PC1 and PC2) into melanocortins and B-endorphin}} |
|
| |
|
| POMC was first noted for its importance in appetite and obesity in rodent studies. POMC-null mice were seen to exhibit [[hyperphagia]] and [[obesity]] without [[insulin resistance]]. Their [[adrenal gland]] was also atrophic and [[glucocorticoid]] levels were undetectable as a result of a lack of [[ACTH]] stimulating adrenal cell secretion and proliferation. This suggestion that POMC has a role in energy balance was supported by studies on rare individuals with POMC mutations. In humans, a lack of POMC is fatal unless glucocorticoids are administered from birth as [[cortisol]] is essential for humans. A few rare individuals with the mutation have shown very similar phenotype to the POMC knockout mouse. | | PC1 and PC2 are expressed in cells other than POMC cells and have important physiological functions other than POMC cleavage. For example, PC1 is essential for the biosynthesis of [[insulin]] and PC2 for the biosynthesis of [[glucagon]]. However, transgenic mice deficient in PC2 (PC2 'knockout' mice) exhibit no α-MSH expression at all, and [[Prader Willi]] patients have reduced hypothalamic PC2 expression. <ref>Millington ''et al.'' (2003)</ref> The PC2 knockout mice are so defective in other ways that it is hard to tell whether the lack of alpha MSH has an effect.<ref>Scamuffa ''et al.'' (2006)</ref> Prader Willi sufferers exhibit an obese phenotype but again this syndrome comes as a result of a mutation of several genes so it cannot be inferred that PC2 mutation is solely responsible for the obese and hyperphagic phenotype. |
|
| |
|
| While these studies show that POMC-derived hormones may have a role in energy balance they don’t tell us which peptides are responsible for the effects and furthermore the lack of adrenal hormones as a secondary result of POMC lack may overshadow the primary POMC effects. Therefore we are required to look at the peptides in more detail. The main system implicated in energy balance is the melanocortin system.
| | Leptin-deficient mice show an upregulation of POMC and PC2 expression. |
|
| |
|
| === Melanocyte-stimulating hormones and their receptors === | | === Melanocortin receptors === |
| This is made up of several endogenous agonists such as α, β, γ MSH and interestingly has two endogenous inverse agonists as well, AgRP and agouti. These act on five different subtypes of the MCR (MCR1-5).
| | {{Image|Melanocortin receptors.JPG|right|500px|'''Melanocortin receptors'''}} |
| | | The melanocortin system encompasses a number of CNS circuits including |
| {{Image|Melanocortin receptors.JPG|centre|700px|'''Melanocortin receptors'''}} | |
| | |
| ==The Melanocortin Pathway==
| |
| The central melanocortin system encompasses a number of CNS circuits including | |
| #α-MSH -containing neurons of the arcuate nucleus. | | #α-MSH -containing neurons of the arcuate nucleus. |
| #POMC neurons in the [[nucleus of the solitary tract]] (NTS) | | #POMC neurons in the [[nucleus of the solitary tract]] (NTS) |
| #AgRP- containing neurones of the arcuate nucleus. AgRP is an inverse agonist at MC4 receptors, thus opposes the actions of α-MSH. AgRP is co-expressed by the orexogenic neurons that make neuropeptide Y (NPY). | | #AgRP- containing neurones of the arcuate nucleus. AgRP is an inverse agonist at MC4 receptors, thus opposes the actions of α-MSH. AgRP is co-expressed by the orexogenic neurons that make [[neuropeptide Y]] (NPY). MC4 agonists reduce food intake and increase energy expenditure, thus reducing body weight, wheras MC4 antagonists enhance food intake (hyperphagia) and decrease energy expenditure, and thus increase body weight. |
| | |
| In addition to its involvement in regulating energy homeostasis, the melanocortin system plays a role in mediating a number of physiological processes within the body including cardiovascular control, inflammation, natriuresis and sexual function.
| |
| | |
| '''Inflammation'''
| |
| α-MSH has potent anti-inflammatory effects although its precise mechanism is not fully understood, it is believed to influence vascular permeability and thus may act by decreasing it.
| |
| | |
| '''Cardiovascular'''
| |
| Central application of γ- MSH (MC3R specific agonist, can induce [[tachycardia]], while the bradychardia that occurs following electrical stimulation of the arcuate nucleus can be inhibited by the administration of SHU-9119 into the DVC. As it acts through MC4R this highlights a potential role for this receptor in mediating cardiovascular control.
| |
| | |
| '''Natriuresis'''
| |
| γ-MSH acts on MC3R in the [[kidney]] to promote [[natriuresis]], and hence a deficiency of this peptide, or a mutation of the receptor results in salt-sensitive hypertension in mice.
| |
| | |
| | |
| '''Sexual Function'''
| |
| Activation of MC4R plays a role in erectile function in males while MC3R is believed to induce [[lordosis]] in females.
| |
| | |
| {{Image|MC4R PATHWAYS.JPG|centre|700px|Circulating molecules cross the blood-brain barrier and signal to the arcuate nucleus the nutritional state of the body. These primary neurones then signal to secondary neurones within the hypothalamus. The cascade of signalling impacts centrally and peripherally to increase food intake or reduce food intake as well as acting on other important contributors that regulating body weight, metabolism, and energy expenditure.}}
| |
| | |
| | | |
| Although the melanocortin system is central to the regulatory mechanisms controlling appetite and satiety, the precise mechanism is not fully understood. Complexity arises from both the direct and indirect effects of a number of compounds including leptin, insulin, glucose, ghrelin, NPY, serotonin, peptide YY and endorphin, all of which act in isolation and in sync to mediate their effects on these POMC neurons. | | Although the melanocortin system is central to the regulatory mechanisms controlling appetite and satiety, the precise mechanism is not fully understood. Complexity arises from both the direct and indirect effects of a number of compounds including leptin, insulin, glucose, ghrelin, NPY, serotonin, peptide YY and endorphin. |
| | |
| Agonists Antagonists
| |
| Reduce food intake Enhance food intake (hyperphagia)
| |
| Increase energy expenditure Decrease energy expenditure
| |
| Reduce body weight Increase body weight
| |
| | |
| | |
| | |
| This highlights the role of the melanocortin system in regulating energy homeostasis, and why disruption in the genes controlling this system, i.e. genetic mutations of the system, can result in individuals which are hyperphagic and consequentially obese.
| |
| | |
| A number of mutations of this system have been identified in mice, all of which show a dysregulation in energy homeostasis. Many of the mutations discovered involve excess production of POMC antagonists, so that melanocortin agonists can’t bind to melanocortin receptors to suppress appetite.
| |
| | |
| #Excess production of AgRP induces its antagonistic effects through binding to both MC1R and MC4R.
| |
| #An increase in the expression of AgRP, which functions by antagonising receptors MC3R and MC4R. This prevents the potent appetite suppressor α-MSH from binding.
| |
| #A mutation which results in a deficiency in the number of MC4R receptors.
| |
| #The insufficient production of POMC derived peptides to bind to these receptors.
| |
| Obese individuals have presented with POMC deleterious gene mutations, as well as with heterozygous mutations in the MC4R receptor.
| |
| | |
| POMC neurons and their peptides mediate satiety signals, while NPY neurons induce hunger signals and decrease energy expenditure. This integration involves both long term signals (leptin from adipose tissue and insulin), as well as acute hunger/satiety signals from the brainstem.
| |
| | |
| The melancortin alpha-MSH is one of the most important regulators of energy homeostasis in the hypothalamus, where it induces a state of satiety within an individual through its actions on the MC4R.
| |
| Additionally, administration of ACTH into certain regions of the hypothalamus has similar effects.
| |
| Agouti is an antagonist at MC1R and MC4R receptors, while AgRP incurs antagonistic effects through its action on MC3R and MC4R receptors. Due to suppression of the alpha MSH anorectic signal, mutant mice with ectopic expression of these peptides are hyperphagic with an increase in adipose mass, lean mass, hyperinsulinemia and consequentially are obese.
| |
|
| |
|
| '''Melanocortins''' are peptides derived from POMC, and are determined by tissue-specific post-translational cleavage. POMC gene expression has been identified in a number of tissues including the pituitary, skin, immune system and hypothalamus, which highlights the many physiological processes that POMC is involved in. POMC and CART are co-expressed in a subpopulation of neurones in the arcuate nucleus, and the expression of both is stimulated by leptin. AgRP and α-MSH are involved in appetite regulation via their actions on MC4R. Melanocortin receptors MC3R and MC4R are unique in that have both endogenous agonists and antagonists. Obesity can arise as a result of a POMC deficiency. Some melancortin receptors have been found on adipocytes (MC4R), suggesting that peripheral detection of these circulating melanocortins may also be involved in regulating energy homeostasis. Expression of these peptides in the anterior pituitary, results in the production of ACTH via the cleavage of POMC by the enzyme prohormone convertase1 (PC1). Stimulation of adrenal steroiogenesis then occurs via the action of ACTH on MC2R.
| | Genetic mutations of the system, can result in individuals which are hyperphagic and consequentially obese. A number of mutations of this system have been identified in mice, all of which show a dysregulation in energy homeostasis. Many of the mutations discovered involve excess production of POMC antagonists, so that melanocortin agonists can’t bind to melanocortin receptors to suppress appetite. |
|
| |
|
| '''Alpha-MSH'''
| | Leptin secreted from [[adipose tissue]] and insulin secreted from the [[pancreas]] also regulate food intake, in part by their actions on the melanocortin systems. Excess adipose tissue (in obese individuals) results in an increase in leptin production, which normally induces a feeling of satiety, but excess production of NPY (as occurs in some mutations of the melancortin system), can suppress its effects. Similarly insulin levels show a marked increase in obese individuals, with hyperinsuliemia being one of the first metabolic disturbances identified in those obese subjects with mutations of the melanocortin system. |
| Alpha-MSH, has been widely accepted as the main endogenous agonist, derived from the POMC gene, which is cleaved in the hypothalamus. It exerts its anorexigenic effects through MC3R and MC4R. In addition to suppressing appetite, alpha- MSH is has been shown to increase both metabolism and body temperature, which is referred to a coordinated catabolic pattern. '''Balasko et.al 2010''' administered i.c.v alpha-MSH to rats and found that in addition to a reduction in food intake, the rats also had an increased metabolic rate.
| |
| | |
| '''Leptin''' is produced primarily in white adipose tissue but also to some extent in brown adipose tissue, stomach, placenta. Numerous leptin receptors have been identified, all of which transmit their signal via the JAK-STAT pathway. The leptin receptor involved in regulating appetite is the receptor type Ob-Rb, which is present in many brain regions including ARC, PVN, DMH and LHA of the hypothalamus. When leptin binds to the Ob-Rb receptor, the subsequent signalling cascade results in a decrease in appetite through inhibition of the peptides involved in inducing orexigenic effects (NPY, MCY, AGRP and orexins). Additionally the anorexigenic peptides peptides (alpha-MSH, CART and CRH) are stimulated. Excess body weight results in excess leptin production from adipose tissue(released in a regular pulsatile manner) and hence leptin can act to restore normal energy homeostasis, by suppressing appetite and increasing energy expenditure through its effect on anorextic peptides.
| |
| | |
| Leptin secreted from adipose tissue and insulin secreted from the pancreas also regulate food intake. Excess adipose tissue (in obese individuals) results in an increase in leptin production (which normally induces a feeling of satiety), but excess production of NPY (as occurs in some mutations of the melancortin system), can suppress its effects. Similarly insulin levels show a marked increase in obese individuals, with hyperinsuliemia being one of the first metabolic disturbances identified in those obese subjects with mutations of the melanocortin system. | |
|
| |
|
| '''Insulin''' | | '''Insulin''' |
| Line 87: |
Line 39: |
|
| |
|
| '''Ghrelin''' | | '''Ghrelin''' |
| [[Ghrelin]] stimulates arcuate nucleus production of NPY and AgRP, while inhibiting the suppressive effects that leptin induces on appetite. Ghrelin administration in rats produces hyperphagia and increased body weight.Central administration of ghrelin results in excessive eating, accompanied by increased arcuate NPY and AgRP expression. | | [[Ghrelin]] stimulates arcuate nucleus production of NPY and AgRP, while inhibiting the suppressive effects that leptin induces on appetite. Ghrelin administration in rats produces hyperphagia and increased body weight. Central administration of ghrelin results in excessive eating, accompanied by increased arcuate NPY and AgRP expression. |
| | |
| An example of the complexity that arises in fully understanding the precise mechanism governing this system arises from mice AgRP KO models. Interestingly, while the involvement of AgRP in the melanocortin system is undisputed, one would expect such KO to present with altered phenotypes and eating patterns, yet these AgRP were just like their wild type counterparts.
| |
| NPY has been identified as one of the most abundant and potent orexigenic peptides of the hypothalamus. While ghrelin ahs short term effects, administration of NPY produces a prolonged and substantial increase in food consumption and a decrease in thermogenesis, resulting in an overall increase in body weight and leading to obesity. NPY exerts its action via G- protein coupled receptors, 5 of which have been identified, of which Y5 has been identified as being involved in mediating its effect on feeding. Circulating levels of leptin and insulin can regulate NPY synthesis, while glucocorticoids serve to stimulate it. During times of fasting, low levels of insulin and leptin result in the up-regulation of NPY synthesis. Interestingly, an obvious increase in ARC NPY neuronal activity is not recorded in diet induced obese individuals, which suggests that it may be involved in ensuring a certain amount of energy stores, but when this store is in excess, NPY neurons exhibit diminished activity, potentially in an effort to restore normal energy homeostasis.
| |
| | |
| '''AgRP''' AgRP inhibits the effects of α-MSH on inducing its anorexigeneic effect. AGRP is potent in activating appetite through its anatagonistic activity on both MC3R and MC4R and is expressed only in the arcuate nucleus of the hypothalamus All neurons expressing AgRP also produce NPY, and project to various brain sites including PVN and DMH. Leptin inhibits the expression of AgRP, while periods of starvation result in an increase in its expession. Unlike NPY which induces strong, short lived effects on stimulating appetite, AgRP administration can induce hyperphagia for up to one week.
| |
| | |
| Thus, when energy stores are low, there is reduced leptin released from adipose tissue, which in turn allows in an increase in the production of orexigenic peptides including NPY, AgRP and a decrease in alpha-MSH and CART. The opposite is true in times if positive energy imbalance.
| |
| | |
| Although Peptide YY is an appetite suppressor, it appears to mediate its effects through a distinct pathway that does not involve the melanocortin system as both POMC and MC4R KO mice continued to show a decrease in food intake following its administration, indicating that another system may be involved. This further highlights the complexity of the mechanisms controlling energy homeostasis.
| |
| | |
| == Experimental evidence and methods used to investigate melanocortin ==
| |
| To date there are several human and animal experimental procedures that exist and show defects in the melanocortin system. Below are a few examples of said experiments taken from and simplified from scientific papers:
| |
| | |
| ===1. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models===
| |
| Kate L.J Ellacott and Roger D Cone 2006
| |
| | |
| a. The focus of this paper lies on the central nervous system and MC4 receptor
| |
| | |
| b. The agouti mouse is one of the oldest genetic models of obesity. Two different strains of the mouse exist:
| |
| i. Lethal yellow = Ay
| |
| ii. Viable yellow = Avy
| |
| | |
| c. The agouti mouse is set apart by hyperinsulinemia, hyperphagia, hypometabolism and increased linear growth compared to wild type mouse
| |
| | |
| d. Agouti = endogenous melanocortin antagonist, that acts at the MC1-R and MC4-R
| |
| | |
| e. Ay mouse has expression of the agouti antagonist due to deletion of the promoter along with the coding region of the nearby Raly gene
| |
| i. Results in agouti being expressed in all tissues
| |
| ii. Embryonically lethal in homozygous state
| |
| iii. BUT heterozygous Ay mouse survives and is used for research
| |
| | |
| f. Avy mouse has overexpression of the agouti antagonist and appears with a black/yellow agouti colouring compared to the vivid yellow of the Ay mouse.
| |
| | |
| g. When the heterozygous lethal yellow mouse is crossed with the ob/ob mouse the result is a heavier mouse than the individually mutated mice
| |
| | |
| i. Proposes that the central melanocortin system is independent and surplus of the leptin pathway
| |
| | |
| h. Melanocortin system has been indicated to also play a role in:
| |
| | |
| i. Mediating hunger
| |
| ii. Mediating satiety
| |
| iii. Meal termination
| |
| iv. Meal initiation
| |
| v. Hunger cues
| |
| i. 1997 = creation of the MC4-R null mouse
| |
| i. Repeated the agouti mouse phenotype and resulted in melanocortin obesity syndrome
| |
| | |
| j. Haploinsufficiency at MC4-R = 5% of cases of severe obesity in children
| |
| | |
| k. The obesity seen in MC4-R null mice appears to be similar to the metabolic syndrome seen in humans
| |
| | |
| | |
| ===2. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R)===
| |
| Daniel L. Marks, Victor Hruby, Gregor Brookhart, and Roger D. Cone
| |
| | |
| a. This paper focuses on the hypothalamic melanocortin system through the activation of MC4-R
| |
| | |
| b. Mice were obtained and raised in a 12 hour light/dark cycle. They were housed individually and food intake measured
| |
| | |
| c. Male animals were used – 6-10 weeks old
| |
| | |
| d. d-Trp8-γ-MSH ( an injection of compounds that should act on the MC3-R to inhibit POMC neuronal activity and ultimately encourage feeding) was dissolved and administered with control mice receiving a saline injection minus the dissolved d-Trp8-γ-MSH in order to prove that any results were not caused by stress
| |
| | |
| e. The animals food intake was measured and compared via:
| |
| i. d-Trp8-γ-MSH injection vs. no d-Trp8-γ-MSH injection
| |
| ii. light vs dark
| |
| | |
| f. They found that MC3-R acts as an inhibitory auto receptor in POMC neurons
| |
| | |
| ===3. A Role for the Endogenous Opioid ß-Endorphin in Energy Homeostasis.===
| |
| Suzanne M. Appleyard, Michael Hayward, Juan I. Young, Andrew A. Butler, Roger D. Cone, Marcelo Rubinstein and Malcolm J. Low
| |
| | |
| a. The idea of PMOC neurons in the hypothalamic arcuate nucleus is focused on in this paper and their role in metabolism and balance.
| |
| | |
| b. They discuss Beta-Endorphin which has a high affinity for certain subtypes of opioid receptors
| |
| | |
| c. Mice were weighed weekly from 3 weeks of age
| |
| | |
| d. At 5 months they were euthanized and their liver, speen, kidney, heart, testis and any other fat pads removed and weighed
| |
| | |
| e. They were housed alone as of 5 weeks of age
| |
| | |
| f. They were allowed food at own pleasure and an average intake of food was calculated
| |
| | |
| g. Oxygen consumption was also recorded
| |
| | |
| h. After a 16h -20h fast the mice were killed. The following was measured and recorded:
| |
| i. Insulin
| |
| ii. Leptin levels
| |
| iii. Glucose from blood
| |
| | |
| i. To measure glucose tolerance during when the mouse was alive they were injected with 2g of glucose after a 16h fast and tail bleeds were recorded
| |
| | |
| j. Beta-endorphin levels were recorded from the hypothalamus and the pituitary
| |
| k. From the above experiments it was determined that mice lacking beta-endorphin were heavier and greater adiposity than wild type mice
| |
| | |
| As you can see from the experiments shown and explained above, it is clear that several defects in the melanocortin system exist. There are few human examples to date, as most experiments are yet to overcome the animal models and move onto humans.
| |
| | |
| == Suggested future studies ==
| |
| Despite the importance of melanocortins for appetite regulation, much of the underlying molecular mechanisms that facilitate the melanocortin signalling pathways remain unidentified.
| |
| | |
| '''Melanocortin Receptors G-proteins: A question of cross-talk?'''
| |
| POMC expression is up-regulated in α-MSH neurones as result of increased leptin and insulin signalling. This has been proposed to sequentially increase α-MSH release which acts predominantly through activation of the G-protein coupled receptors MC3R and MC4R which are Gs linked receptors. Gs stimulate the up-regulation of adenylase cyclase and thus increase cAMP levels, and a multidude of sequential kinase signalling resulting in a complex cascade of second messengers that facilitate its anorexigenic effects and prevent orexigenic pathways.
| |
| | |
| Recent studies have unveiled the possibility for the involvement of alternative G-proteins or the coupling of G-proteins to create a specific effect. However, there are contradictory studies between the involvement of Gq’s and the activation of PLC and calcium signal which has been seen in some studies but not in others however, the cell lines used were different and furthermore the results are yet to be confirmed in vivo <ref name=Breit10> Breit A ''et al.'' (2010) Alternative G protein coupling and biased agonism: New insights into melanocortin-4 receptor signalling. ''Mol Cell Endocrinol'' doi:10.1016/j.mce.2010.07.007</ref> <ref>Biaoxin C ''et al.'' (2006) Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase ''Peptides'' 27: 2846-57 doi:10.1016/j.peptides.2006.05.005</ref>. Additionally, another study found that the inhibition of Gi/o specific cells expressing MC4R treated with agonist created a suppression of ERK1/2 phosphorylation indicating a possible role for Gi’s and downstream PKC/calcium activation of ERK 1/2 as opposed to conventional Gs PKA signalling initially described in certain cell types <ref name=Breit10/>. Clearly, these areas of research is open to clarification in terms of the G-proteins involved in melanocortin signalling and activation of important downstream activity of molecules such as ERK 1/2 and thus enable us to fine tune the understanding of appetite regulation.
| |
| | |
| '''αMSH: Please release me let me go'''
| |
| Although it is thought that leptin and insulin are responsible for increasing POMC expression and consequently α-MSH release, little is understood, on the molecular level, of how and where α-MSH is actually released. There is very little evidence of studies into the process by which it is exocytosed and more research into the storage and release mechanisms of α-MSH is required to understand the αMSH pathway and bring insight into any other possible means of targeting this pathway.
| |
| | |
| '''Melanocortin 3 Receptor (MC3R)'''
| |
| Although the MC3R is implicated in appetite regulation it does not have such in-depth association with obesity as the MC4R has. However, its involvement should not be overlooked, as studies have shown that mutations, heterozygous genotype and knock out experiments can create the pathophysiological state or at least predispose humans and murine species to obesity either in childhood or later in life, especially apparent when coupled to an obesigenic environment <ref name=Mountjoy10> Mountjoy K. (2010) Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes ''Biochem J'' 428:305-24 doi:10.1042/BJ20091957 305</ref>. Moreover, there is confusion as to the roles that this receptor has in an anorexic effect since it is also thought that it is part of the auto-regulation of POMC providing negative feedback. Studies of MC3R knockout mice show primarily alterations to in metabolic syndromes whereas MC4R alters feeding behaviour and intake as well as energy expenditure <ref>Butler AA, Cone RD (2003) Knockout studies defining different roles for melanocortin receptors in energy homeostasis ''Ann NY Acad Sci'' 994:240-5</ref>.
| |
| | |
| '''Agouti-related protein signalling: antagonist or inverse agonist?'''
| |
| AgRP is thought to have a competitive antagonistic role on MCR to prevent αMSH signalling however, AgRP can produce autonomous orexigenic effects by reducing the cAMP increase caused by binding of αMSH potentially through Gi signalling suggesting that it has inverse agonist qualities. This is primarily observed in terms of its ability to reduce cAMP levels <ref>Nijenhuis WAJ ''et al.'' (2001) AgRP(83–132) acts as an inverse agonist on the human melanocortin-4 receptor ''Mol Endocrinol'' 15:164–71</ref>. Recent studies have reiterated these findings for a AgRP's role as an inverse agonist ''in-vivo'' as well as eluding to recent studies in MC4R knock out mice in which AgRP induced hyperphagic state was still observed suggestive of its action through a receptor independent of the melanocortin receptors <ref>Tolle V, Low MJ (2008) In Vivo evidence for inverse agonism of agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice ''Diabetes''57:86-94</ref>. The exact composition of MCRs and the complexes that are formed at the membrane is an avenue that requires exploration especially since the picture is complex; MCR could potentially interact with a variety of G-proteins, accessory proteins such as syndecans, mahoganoid and especially MRAPs with MC4R to manipulate various aspects of αMSHs signalling capacity <ref name=Mountjoy10/>.
| |
| | |
| '''Lipid rafts'''
| |
| The cholesterol composition of the lipid membrane affects cAMP signalling, with higher levels dampening the cAMP response because the lipid rafts segregate the G-protein signalling components accessibility to each other. Accordingly, the lipid micro-domain surrounding the MCR’s within the membrane may alter the result of α-MSH signalling to produce cell-specific effects.<ref name=Breit10/>.
| |
| | |
| '''A role for BNDF/Trk signalling'''
| |
| The neurotrophic [[brain-derived neuronal growth factor]] (BNDF) acts through the [[tropomysin receptor kinase B]] (Trk) and is thought to be activated downstream of melanocortin signalling through MC4R. This is proposed to be due to the ability of synthetic [[mineralocorticoid]]s to promote BDNF expression within the [[ventromedial nucleus]] of the hypothalamus (VMH), in addition to the ability of BDNF to blunt feeding in MC4R knockout mice <ref>Oswal A, Yeo GS (2007) The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics ''Obesity Rev'' 8:293-306</ref>. However, how they interact is not clear and further investigation at the molecular level will shine light on the downstream signalling involved through protein-protein interaction assay techniques such as a GST protein interaction pull down of the 'bait' protein, possibly the section of protein thought most likely to interact with another protein, and wash with cell lysates containing potential 'prey' molecule candidates followed by immunoblotting to assess interactions <ref>Thermo Fisher Scientific Inc. (2010) Pierce GST and His Tag Protein Interaction Pull-Down Kitshttp://www.piercenet.com/products/browse.cfm?fldID=42E8E027-6231-4512-8DDE-BB026740966B</ref>.
| |
|
| |
|
| '''Thyroid hormone''' | | '''Thyroid hormone''' |
| MC4R is expressed in a variety of neuronal cell types, including the [[thyrotropin-releasing hormone]] (TRH) neurones of the PVN, consistent with a role in metabolism and thus energy homeostasis. Inhibitory feedback from [[thyroxin]]'s (T4) biologically active derivative [[triodothyronine]] (T3)is occurring at the MC4R <ref>Decherf S ''et al.'' (2010) Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression ''PNAS'' 107:4471–6</ref>.This incidentally alleviates inhibition of food intake and allows the activation of orexigenic pathways. The negative feedback is strongest in the brainstem region where it is thought to regulate meal size and [[thermogenesis]]. | | MC4R is expressed in a variety of neuronal cell types, including the [[thyrotropin-releasing hormone]] (TRH) neurones of the PVN, consistent with a role in metabolism and thus energy homeostasis. Inhibitory feedback from [[thyroxin]]'s (T4) biologically active derivative [[triodothyronine]] (T3)is occurring at the MC4R <ref>Decherf S ''et al.'' (2010) Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression ''PNAS'' 107:4471–6</ref>.This incidentally alleviates inhibition of food intake and allows the activation of orexigenic pathways. The negative feedback is strongest in the brainstem region where it is thought to regulate meal size and [[thermogenesis]]. |
| | |
| '''Corticotropin Releasing Hormone (CRH)'''
| |
| CRH has also been discovered to partake in appetite regulation and studies using double labelling ''in situ'' hybridization have found that MC4R is also expressed by CRH neurons inthe PVN. CRH is one of the elements regulated downstream of MC4R activation and aids arexogenic systems. Consequently, linking the melanocortin pathway to the hypothalamic pituitary axis and thus to an involvement in stress. X. Y Lu ''et. al'' (2003)proposes the implications of this connection to stress as resulting in a relation to eating disorders and therefore is of particular interest not merely in treating anorexia nervosa but in terms of potentially opening an avenue of insight into obesityies polar opposite and help aid the research into the requirements for the 'happy medium'<ref> Xin-Yun L ''et al.'' (2003) Interaction between alph-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-Adrenal Responses ''J Neurosci'' 23:7863–72</ref>. However, more work is required in terms of the exact receptor type that CRH is acting on and also whether the HPA axis requires melanocortin signalling or likewise is the HPA axis required for appropriate melanocortin regulation of feeding?
| |
|
| |
|
| ==References== | | ==References== |
| <references/>
| | {{reflist | 2}}[[Category:Suggestion Bot Tag]] |
Melanocortins are peptides derived from pro-opiomelanocortin (POMC), determined by tissue-specific post-translational cleavage. The regulation of appetite involves a complex interplay between circulating hormones, neurotransmitters, neuropeptides and nutrients, and the melanocortin system is an important component of this. [1][2][3][4][5][6]
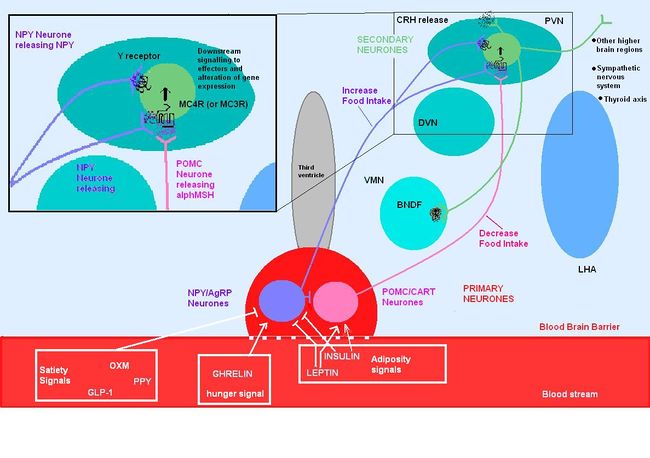
Circulating molecules signal to the arcuate nucleus the nutritional state of the body
POMC is expressed in many tissues including the pituitary gland and the brain, and its products are involved in many different physiological processes. In the brain, POMC is expressed in the arcuate nucleus of the hypothalamus and the nucleus tractus solitarii (NTS) of the caudal brainstem, and products of POMC are important in appetite regulation. POMC was first noted for its importance in appetite and obesity in rodent studies. POMC-null mice exhibit hyperphagia and obesity. The suggestion that POMC has a role in appetite and energy balance is supported by studies on rare individuals with POMC mutations. In humans, a lack of POMC is generally fatal unless glucocorticoids are administered from birth as cortisol is essential for humans, but a few rare individuals with the mutation have shown very similar phenotype to the POMC knockout mouse. [7][8]
POMC expression in the arcuate nucleus is regulated by leptin, a hormone secreted by adipose tissue. Both the opioid peptide B endorphin and the melanocortin alpha-melanocyte stimulating hormone (α-MSH) are cleaved from POMC; α-MSH is a very potent inhibitor of feeding behaviour, but β endorphin increases feeding behaviour (especially of highly palatable foods). It is therefore important to explore how the prohormone may be processed in different tissues and what dictates this. In the same sense, how are the cleavage enzymes regulated to produce various concentrations of the different peptide hormones? In the brain, the actions of α-MSH are mediated via specific melanocortin receptors - particulary MC3 and MC4 receptors. These receptors are unusual in that they have both endogenous agonists and antagonists.
Agouti and agouti-related peptide
The central melanocortin system involves several agonists such as α, β, γ MSH and two inverse agonists, agouti and AgRP. These act on five subtypes of MC receptors (MCR1-5). [9]
Of these, AgRP and α-MSH are thought to be most important for appetite regulation mainly via their actions on MC4 receptors. In addition to suppressing appetite, α-MSH increases both metabolism and body temperature. [10]Some MC4 receptors have been found on adipocytes, suggesting that circulating melanocortins may also be involved in regulating energy homeostasis.
Agouti is an antagonist at MC1 and MC4 receptors, while AgRP iis an inverse agonist at MC3 and MC4 receptors. Mutant mice with ectopic expression of these peptides are hyperphagic with an increase in adipose mass, lean mass, and hyperinsulinemia. [11]
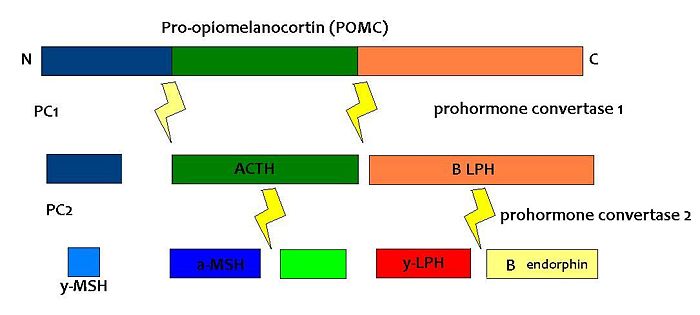
(PD) Image: Jessica Ivy The breakdown of POMC by prohormone convertase enzymes (PC1 and PC2) into melanocortins and B-endorphin
PC1 and PC2 are expressed in cells other than POMC cells and have important physiological functions other than POMC cleavage. For example, PC1 is essential for the biosynthesis of insulin and PC2 for the biosynthesis of glucagon. However, transgenic mice deficient in PC2 (PC2 'knockout' mice) exhibit no α-MSH expression at all, and Prader Willi patients have reduced hypothalamic PC2 expression. [12] The PC2 knockout mice are so defective in other ways that it is hard to tell whether the lack of alpha MSH has an effect.[13] Prader Willi sufferers exhibit an obese phenotype but again this syndrome comes as a result of a mutation of several genes so it cannot be inferred that PC2 mutation is solely responsible for the obese and hyperphagic phenotype.
Leptin-deficient mice show an upregulation of POMC and PC2 expression.
Melanocortin receptors
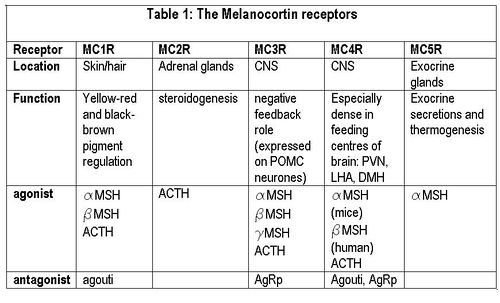
(PD) Image: Jessica Ivy Melanocortin receptors The melanocortin system encompasses a number of CNS circuits including
- α-MSH -containing neurons of the arcuate nucleus.
- POMC neurons in the nucleus of the solitary tract (NTS)
- AgRP- containing neurones of the arcuate nucleus. AgRP is an inverse agonist at MC4 receptors, thus opposes the actions of α-MSH. AgRP is co-expressed by the orexogenic neurons that make neuropeptide Y (NPY). MC4 agonists reduce food intake and increase energy expenditure, thus reducing body weight, wheras MC4 antagonists enhance food intake (hyperphagia) and decrease energy expenditure, and thus increase body weight.
Although the melanocortin system is central to the regulatory mechanisms controlling appetite and satiety, the precise mechanism is not fully understood. Complexity arises from both the direct and indirect effects of a number of compounds including leptin, insulin, glucose, ghrelin, NPY, serotonin, peptide YY and endorphin.
Genetic mutations of the system, can result in individuals which are hyperphagic and consequentially obese. A number of mutations of this system have been identified in mice, all of which show a dysregulation in energy homeostasis. Many of the mutations discovered involve excess production of POMC antagonists, so that melanocortin agonists can’t bind to melanocortin receptors to suppress appetite.
Leptin secreted from adipose tissue and insulin secreted from the pancreas also regulate food intake, in part by their actions on the melanocortin systems. Excess adipose tissue (in obese individuals) results in an increase in leptin production, which normally induces a feeling of satiety, but excess production of NPY (as occurs in some mutations of the melancortin system), can suppress its effects. Similarly insulin levels show a marked increase in obese individuals, with hyperinsuliemia being one of the first metabolic disturbances identified in those obese subjects with mutations of the melanocortin system.
Insulin
After a meal, there is a dramatic increase in insulin levels, some of which crosses the blood-brain barrier in a concentration that is representative of circulating insulin levels. Central administration of insulin acts on both NPY and POMC systems, and increases POMC mRNA synthesis as well as inhibiting food intake in fasting rats. Rats with untreated diabetes have a diminished amount of POMC mRNA, which is indicative of its involvement in stimulating its synthesis.
Ghrelin
Ghrelin stimulates arcuate nucleus production of NPY and AgRP, while inhibiting the suppressive effects that leptin induces on appetite. Ghrelin administration in rats produces hyperphagia and increased body weight. Central administration of ghrelin results in excessive eating, accompanied by increased arcuate NPY and AgRP expression.
Thyroid hormone
MC4R is expressed in a variety of neuronal cell types, including the thyrotropin-releasing hormone (TRH) neurones of the PVN, consistent with a role in metabolism and thus energy homeostasis. Inhibitory feedback from thyroxin's (T4) biologically active derivative triodothyronine (T3)is occurring at the MC4R [14].This incidentally alleviates inhibition of food intake and allows the activation of orexigenic pathways. The negative feedback is strongest in the brainstem region where it is thought to regulate meal size and thermogenesis.
References
- ↑ Cone R (2006) Studies on the Physiological functions of the melanocortin system Endocrine reviews 27:736-49
- ↑ Qian G, Tamas H (2007) Neurobiology of feeding and energy expenditure Annu Rev Neurosci 30:367-98
- ↑ Seeley R et al. (2004) The critical role of The melanocortin system in the control of energy balance Annu Rev Nutrition 24:133-49
- ↑ Mountjoy K (2010) Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes Biochem J 428:305-24
- ↑ Fan W et al. (2000) The central melanocortin system can directly regulate serum insulin levels Endocrinology 141:3072-9
- ↑ Gantz I, Fong TM (2003) The melancortin system Am J Physiol 284:E468-74
- ↑ Oswal A, Yeo GSH (2007) The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics Obesity Rev 8:293–306
- ↑ Farooqi IS, O’Rahilly S(2008) Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. nature clinical practice Endocrinol Metabol
- ↑ Wikberga, J et al. (2000) New Aspects on the melanocortins and their receptors Pharmacol Res 42: 393-420
- ↑ Balasko et al. (2010) Central alpha-MSH, energy balance, thermal balance and antipyresis J Thermal Biol 35:211-7
- ↑ Pritchard L et al.(2002) Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity J Endocrinol 172:411-21
- ↑ Millington et al. (2003)
- ↑ Scamuffa et al. (2006)
- ↑ Decherf S et al. (2010) Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression PNAS 107:4471–6


