Flash evaporation: Difference between revisions
imported>Milton Beychok (CZ links) |
mNo edit summary |
||
| (19 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
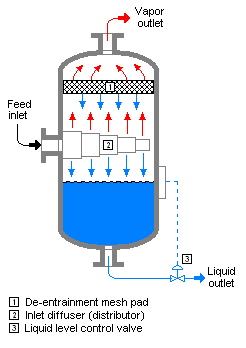
[[Image:Vapor-Liquid Separator.png|frame|right|246px||{{#ifexist:Template:Vapor-Liquid Separator.png/credit|{{Vapor-Liquid Separator.png/credit}}<br/>|}}Typical flash drum or gas-liquid separator]] | [[Image:Vapor-Liquid Separator.png|frame|right|246px||{{#ifexist:Template:Vapor-Liquid Separator.png/credit|{{Vapor-Liquid Separator.png/credit}}<br/>|}}Typical flash drum or gas-liquid separator]] | ||
'''Flash evaporation''' is the partial or total [[vaporization]] that occurs when a [[Boiling point|saturated liquid]] stream undergoes a reduction in [[pressure]] by passing through a throttling valve or other throttling device. This process is one of the simplest [[unit operation]]s. If the throttling valve or device is located at the entry into a [[pressure vessel]] so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a [[flash drum]]. | '''Flash evaporation''' is the partial or total [[vaporization]] that occurs when a [[Boiling point|saturated liquid]] stream undergoes a reduction in [[pressure]] by passing through a throttling valve or other throttling device. This process is one of the simplest [[unit operation]]s. If the throttling valve or device is located at the entry into a [[pressure vessel]] so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a [[flash drum]]. | ||
| Line 10: | Line 8: | ||
==Flash evaporation of a single-component liquid== | ==Flash evaporation of a single-component liquid== | ||
The flash evaporation of a single-component liquid is an [[isenthalpic]] (i.e., constant [[enthalpy]]) process and is often referred to as an ''[[Adiabatic process|adiabatic]] flash'', a ''flash distillation'' or a ''throttling expansion''. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized.<br><br> | The flash evaporation of a single-component liquid is an [[isenthalpic]] (i.e., constant [[enthalpy]]) process and is often referred to as an ''[[Adiabatic process|adiabatic]] flash'', a ''flash distillation'' or a ''throttling expansion''. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized.<br><br> | ||
| Line 63: | Line 60: | ||
==Equilibrium flash of a multi-component liquid== | ==Equilibrium flash of a multi-component liquid== | ||
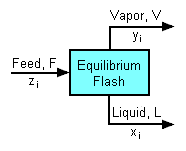
{{Image|Equilibrium Flash.png|right|186px|Schematic of equilibrium flash of a multicomponent feed.}} | |||
The '''equilibrium flash''' of a multi-component liquid is also an isenthalpic process and may be visualized as a simple distillation process using a single [[equilibrium stage]]. It is very different and more complex than the flash evaporation of single-component liquid. For a multi-component liquid, calculating the amounts of flashed vapor and residual liquid in equilibrium with each other at a given temperature and pressure requires a trial-and-error [[Iterative method|iterative]] solution. Such a calculation is commonly referred to as an ''equilibrium flash calculation''. It involves solving the following '''Rachford-Rice equation''':<ref> | |||
{{cite book|author=Harry Kooijman and Ross Taylor|title=The ChemSep Book|edition=2nd Edition|publisher=|year=2000|id=ISBN 3-8311-1068-9}} Also [http://www.chemsep.com/downloads/docs/book2.pdf The ChemSep Book] See pdf page 186 of 194 pdf pages.</ref><ref>[https://www.e-education.psu.edu/png520/m13_p2.html Module Elementary Vapor-Liquid Equilibrium] (Pennsylvania State University)</ref><ref>[http://www.infochemuk.com/publicat/poster.pdf Automatic Plotting of Multiple Phase Boundaries and Flash Calculations], Infochem Computer Services, [[United Kingdom]]</ref><ref>[http://www.mathworks.com/matlabcentral/fileexchange/loadFile.do?objectId=9341&objectType=file Flash Calculations using the Soave-Redlich-Kwong equation of state] (view full-size image)</ref> | |||
:<math>\sum_i\frac{z_i\, (K_i - 1)}{1 + \alpha\, (K_i - 1)}=0</math> | |||
:<math>\sum_i\frac{z_i\, (K_i - 1)}{1 + | |||
{| border="0" cellpadding="2" | |||
|- | |- | ||
|align=right|where: | |align=right|where: | ||
| | | | ||
|- | |- | ||
!align=right|<math> | !align=right|<font style="vertical-align:-30%;"><math>z_i \,</math></font> | ||
|align=left|= | |align=left|= [[mole fraction]] of component <math>i</math> in the feed liquid | ||
|- | |- | ||
!align=right|<math> | !align=right|<font style="vertical-align:-10%;"><math>\alpha</math></font> | ||
|align=left|= | |align=left|= mole fraction of feed that is vaporized = <math>V/F</math> | ||
|- | |- | ||
!align=right|<font style="vertical-align:- | !align=right|<font style="vertical-align:-15%;"> <math>K_i</math></font> | ||
|align=left|= | |align=left|= [[vapor-liquid equilibrium constant]] = <math> y_i / x_i </math> | ||
|- | |- | ||
!align=right|<math> | !align=right|<font style="vertical-align:-30%;"><math>y_i </math></font> | ||
|align=left|= | |align=left|= mole fraction of component <math>i</math> in the flashed vapor | ||
|- | |- | ||
!align=right|<font style="vertical-align:-30%;"><math>x_i </math></font> | |||
|align=left|= mole fraction of component <math>i</math> in the residual liquid | |||
!align=right|<font style="vertical-align:-30%;"><math>x_i | |||
|align=left|= | |||
|} | |} | ||
[[Newton's method]] (also known as the ''[[Newton-Raphson method]]'') is an efficient iterative [[algorithm]] for solving the Rachford-Rice equation. Alternatively, the [[bisection method]] or the [[Brent method]] may be used. Once the equation has been solved for <font style="vertical-align:-7%;"><math>a</math></font>, the compositions <font style="vertical-align:-14%;"><math>x_i</math></font> and <font style="vertical-align:-14%;"><math>y_i</math></font> can be immediately calculated as: | |||
:<math>x_i = \frac{z_i}{1 + \alpha\, (K_i - 1)}</math> | |||
:<font style="vertical-align:-40%;"><math>y_i = K_i\,x_i</math></font> | |||
The equilibrium flash of multi-component liquids is very widely utilized in [[Petroleum refining processes|petroleum refineries]], [[petrochemical]] and [[chemical plant]]s and [[natural gas processing]] plants. | The equilibrium flash of multi-component liquids is very widely utilized in [[Petroleum refining processes|petroleum refineries]], [[petrochemical]] and [[chemical plant]]s and [[natural gas processing]] plants. | ||
==Spray drying== | ==Spray drying and freeze drying== | ||
[[Spray drying]] is a means for rapid drying of a [[solution]] or a [[slurry]] of very small solid particles suspended in a liquid. The feed solution or slurry is first [[atomization|atomized]] into very small liquid droplets in the presence of a stream of hot dry air. The liquid rapidly evaporates leaving behind dry powder or dry solid granules depending on the original droplet size. The dry solids are recovered from the exhaust air by using [[cyclone (industry)|cyclones]], [[Filtration|bag filters]] or [[electrostatic precipitator]]s. | |||
A brief explanation of spray drying has been included here because some readers may consider spray drying to be a form of flash evaporation. However, although it is a form of liquid evaporation, it is quite different from flash evaporation. | |||
[[Freeze drying]] (often referred to as [[lyophilization]]) is a process typically used to preserve water-containing perishable materials. It involves freezing the material, reducing the surrounding pressure and adding sufficient heat to [[Sublimation|sublime]] the frozen water directly from its solid phase to its gas phase. It is a form of induced [[sublimation]] and is very much different than the flash evaporation of a liquid. | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 17 August 2024
Flash evaporation is the partial or total vaporization that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations. If the throttling valve or device is located at the entry into a pressure vessel so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a flash drum.
If the saturated liquid is a single-component liquid (for example, liquid propane or liquid ammonia), a part of the liquid immediately "flashes" into vapor (i.e., evaporates). Both the vapor and the residual liquid are cooled to the saturation temperature of the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional vapor-compression refrigeration systems.
If the saturated liquid is a multi-component liquid (for example, a mixture of propane, isobutane and normal butane), a part of the liquid will also immediately flash into a vapor and the flashed vapor will be richer in the more volatile components than is the remaining liquid.
Flash evaporation of a single-component liquid
The flash evaporation of a single-component liquid is an isenthalpic (i.e., constant enthalpy) process and is often referred to as an adiabatic flash, a flash distillation or a throttling expansion. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized.
- X = 100 ( HuL – HdL ) ÷ ( HdV – HdL )
where: X = weight percent vaporized HuL = upstream liquid enthalpy at upstream temperature and pressure, J/kg HdV
= flashed vapor enthalpy at downstream pressure and corresponding saturation
temperature, J/kgHdL
= residual liquid enthalpy at downstream pressure and corresponding saturation
temperature, J/kg
If the enthalpy data required for the above equation is unavailable, then the following equation may be used.
- X = 100 · cp ( Tu – Td ) ÷ Hv
where: X = weight percent vaporized cp = liquid specific heat at upstream temperature and pressure, J/(kg · °C) Tu = upstream liquid temperature, °C Td = liquid saturation temperature corresponding to the downstream pressure, °C Hv
= liquid heat of vaporization at downstream pressure and corresponding
saturation temperature, J/kg
( Note: The words "upstream" and "downstream" refer to before and after the liquid passes through the throttling valve or device.)
This type of flash evaporation is used in the desalination of brackish water or ocean water by Multi-Stage Flash Distillation. The water is heated and then routed into a reduced-pressure flash evaporation "stage" where some of the water flashes into steam. This steam is subsequently condensed into salt-free water. The residual salty liquid from that first stage is introduced into a second flash evaporation stage at a pressure lower than the first stage pressure. More water is flashed into steam which is also subsequently condensed into more salt-free water. This sequential use of multiple flash evaporation stages is continued until the design objectives of the system are met. A large part of the world's installed desalination capacity uses multi-stage flash distillation. Typically such plants have 24 or more sequential stages of flash evaporation.
Equilibrium flash of a multi-component liquid
The equilibrium flash of a multi-component liquid is also an isenthalpic process and may be visualized as a simple distillation process using a single equilibrium stage. It is very different and more complex than the flash evaporation of single-component liquid. For a multi-component liquid, calculating the amounts of flashed vapor and residual liquid in equilibrium with each other at a given temperature and pressure requires a trial-and-error iterative solution. Such a calculation is commonly referred to as an equilibrium flash calculation. It involves solving the following Rachford-Rice equation:[1][2][3][4]
| where: | |
| = mole fraction of component in the feed liquid | |
| = mole fraction of feed that is vaporized = | |
| = vapor-liquid equilibrium constant = | |
| = mole fraction of component in the flashed vapor | |
| = mole fraction of component in the residual liquid |
Newton's method (also known as the Newton-Raphson method) is an efficient iterative algorithm for solving the Rachford-Rice equation. Alternatively, the bisection method or the Brent method may be used. Once the equation has been solved for , the compositions and can be immediately calculated as:
The equilibrium flash of multi-component liquids is very widely utilized in petroleum refineries, petrochemical and chemical plants and natural gas processing plants.
Spray drying and freeze drying
Spray drying is a means for rapid drying of a solution or a slurry of very small solid particles suspended in a liquid. The feed solution or slurry is first atomized into very small liquid droplets in the presence of a stream of hot dry air. The liquid rapidly evaporates leaving behind dry powder or dry solid granules depending on the original droplet size. The dry solids are recovered from the exhaust air by using cyclones, bag filters or electrostatic precipitators.
A brief explanation of spray drying has been included here because some readers may consider spray drying to be a form of flash evaporation. However, although it is a form of liquid evaporation, it is quite different from flash evaporation.
Freeze drying (often referred to as lyophilization) is a process typically used to preserve water-containing perishable materials. It involves freezing the material, reducing the surrounding pressure and adding sufficient heat to sublime the frozen water directly from its solid phase to its gas phase. It is a form of induced sublimation and is very much different than the flash evaporation of a liquid.
References
- ↑ Harry Kooijman and Ross Taylor (2000). The ChemSep Book, 2nd Edition. ISBN 3-8311-1068-9. Also The ChemSep Book See pdf page 186 of 194 pdf pages.
- ↑ Module Elementary Vapor-Liquid Equilibrium (Pennsylvania State University)
- ↑ Automatic Plotting of Multiple Phase Boundaries and Flash Calculations, Infochem Computer Services, United Kingdom
- ↑ Flash Calculations using the Soave-Redlich-Kwong equation of state (view full-size image)