Vapor-compression refrigeration
Vapor-compression refrigeration[1][2][3] is one of the many refrigeration cycles available for use. It has been and is the most widely used method for air conditioning of large public buildings, private residences, hotels, hospitals, theaters, restaurants and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Petroleum refineries, petrochemical and chemical plant processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize very large-scale vapor-compression refrigeration systems.
Refrigeration may be defined as lowering the temperature of an enclosed space by removing heat from that space and transferring it elsewhere. In more technical terms, it may be defined as a heat pump.
Description of the vapor-compression refrigeration
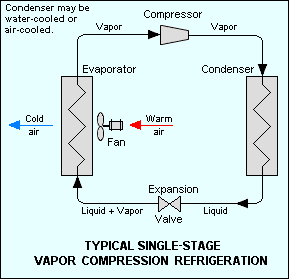
Vapor-compression refrigeration uses a circulating liquid refrigerant as the medium which absorbs and removes heat from the space to be cooled and subsequently rejects that heat elsewhere. Figure 1 depicts a typical vapor-compression system. All such systems have four components: a compressor, a condenser, an expansion valve (also called a throttle valve), and an evaporator.[3] Circulating refrigerant enters the compressor in the thermodynamic state known as a saturated vapor and is compressed to a higher pressure, resulting in a higher temperature as well. The hot, compressed vapor is then in the thermodynamic state known as a superheated vapor and it is at temperature and pressure at which it can be condensed with typically available cooling water or cooling air. That hot vapor is routed through a condenser where it is cooled and condensed into a liquid by flowing through a coil or tubes with cool water or cool air flowing across the coil or tubes. This is where the circulating refrigerant rejects heat from the system and the rejected heat is carried away by either the water or the air (whichever may be the case).
The condensed liquid refrigerant, in the thermodynamic state known as a saturated liquid, is next routed through an expansion valve where it undergoes an abrupt reduction in pressure. That pressure reduction results in the adiabatic flash evaporation of a part of the liquid refrigerant. The auto-refrigeration effect of the adiabatic flash evaporation lowers the temperature of the liquid and vapor refrigerant mixture to where it is colder than the temperature of the enclosed space to be refrigerated.
The cold mixture is then routed through the coil or tubes in the evaporator. A fan circulates the warm air in the enclosed space across the coil or tubes carrying the cold refrigerant liquid and vapor mixture. That warm air evaporates the liquid part of the cold refrigerant mixture. At the same time, the circulating air is cooled and thus lowers the temperature of the enclosed space to the desired temperature. The evaporator is where the circulating refrigerant absorbs and removes heat which is subsequently rejected in the condenser and transferred elsewhere by the water or air used in the condenser.
To complete the refrigeration cycle, the refrigerant vapor from the evaporator is again a saturated vapor and is routed back into the compressor.
Note: Saturated vapors and saturated liquids are vapors and liquids at their saturation temperature and saturation pressure. A superheated vapor is at a temperature higher than the saturation temperature corresponding to its pressure.
Thermodynamic analysis
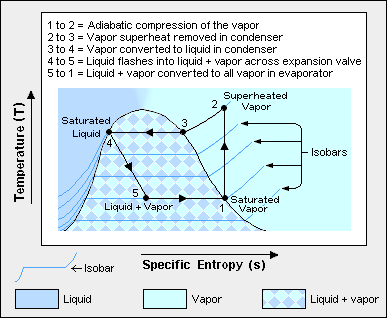
The thermodynamics of the vapor compression cycle can be analyzed on a temperature versus entropy diagram[1][3] as depicted in Figure 2. At point 1 in the diagram, the circulating refrigerant enters the compressor as a saturated vapor. From point 1 to point 2, the vapor is isentropically compressed (i.e., compressed at constant entropy) and exits the compressor as a superheated vapor.
From point 2 to point 3, the superheated vapor travels through part of the condenser which removes the superheat by cooling the vapor. Between point 3 and point 4, the vapor travels through the remainder of the condenser and is condensed into a saturated liquid. The condensation process occurs at essentially constant pressure. The condensation process is where heat is rejected from the system by transfer to the air or water used to condense the vapor.
Between points 4 and 5, the saturated liquid refrigerant passes through the expansion valve and undergoes an abrupt decrease of pressure. That process results in the adiabatic flash evaporation and auto-refrigeration of a portion of the liquid (typically, less than half of the liquid flashes). The adiabatic flash evaporation process is isenthalpic (i.e., occurs at constant enthalpy).
Between points 5 and 1, the cold and partially vaporized refrigerant travels through the coil or tubes in the evaporator where it is totally vaporized by the warm air (from the space being refrigerated) that a fan circulates across the coil or tubes in the evaporator. The evaporator is where heat is absorbed from the space being cooled by transfer from the warm air in the space.
The evaporator operates at essentially constant pressure. The resulting saturated refrigerant vapor returns to the compressor inlet at point 1 to complete the thermodynamic cycle.
It should be noted that the above discussion is based on the ideal vapor-compression refrigeration cycle which does not take into account real world items like frictional pressure drop in the system, slight internal irreversibility during the compression of the refrigerant vapor, or non-ideal gas behavior (if any).
Refrigerants
"Freon" is a trade name for a family of haloalkane refrigerants manufactured by DuPont and other companies. These refrigerants were commonly used due to their superior stability and safety properties: they were not flammable nor obviously toxic as were the fluids they replaced.
Unfortunately, the chlorine-bearing haloalkanes reach the upper atmosphere when they escape. In the stratosphere, chlorofluorocarbons (CFCs) break up due to UV-radiation, releasing their chlorine atoms. These chlorine atoms act as catalysts in the breakdown of ozone, which damages the ozone layer that shields the Earth's surface from the Sun's strong UV radiation. The chlorine will remain active as a catalyst until and unless it binds with another particle, forming a stable molecule. CFC refrigerants in common but receding usage include those named R-11 and R-12.
Newer and more environmentally-safe refrigerants include hydrochlorofluorocarbons (HCFCs) such as chlorodifluoromethane, known as R-22, used in most homes today and hydrofluorocarbons (HFCs) such as R-134a, used in most cars. These have replaced most CFC use. HCFCs in turn are being phased out under the Montreal Protocol and replaced by HFCs, such as R-410A, which lack chlorine.
Newer refrigerants are currently the subject of research, such as supercritical carbon dioxide, known as R-744. These have similar efficiencies compared to existing CFC and HFC based compounds.
Most large-scale industrial refrigeration systems do not use haloalkanes. Instead, they use ammonia or hydrocarbons such as methane, ethane, propane or butane.
Refrigerants used for other than large industrial systems
| Refrigeration application | Short descriptions | Typical refrigerants used |
|---|---|---|
| Domestic refrigeration | Appliances used for keeping food in dwelling units | R-600a, R-134a |
| Commercial refrigeration | Holding and displaying frozen and fresh food in retail outlets | R-134a, R-404A, R-507 |
| Food processing and cold storage | Equipment to preserve, process and store food from its source to the wholesale distribution point | R-134a, R-407C, R-410A, R-507 |
| Transport refrigeration | Equipment to preserve and store goods, primarily foodstuffs, during transport by road, rail, air and sea | R-134a, R-407C, R-410A |
| Electronic cooling | Low-temperature cooling of CMOS circuitry and other components in large computers and servers | R-134a, R-404A, R-507 |
Miscellany
The refrigeration system shown in Figure 1 does not include other equipment items usually provided in a large commercial or industrial vapor compression refrigeration system, such as:
- A pressure vessel, equipped internally with a demister, between the evaporator and the compressor inlet to capture and remove any residual, entrained liquid in the refrigerant vapor because liquid may damage the compressor. Such vapor-liquid separators are often referred to as "suction line accumulators". (In large industrial processes, they are called "compressor suction drums" or "knockout drums".)
- Large commercial or industrial refrigeration systems may have multiple expansion valves and evaporators in order to refrigerate a number of enclosed spaces or rooms. In such systems, the condensed liquid refrigerant may be routed into a pressure vessel, called a receiver, from which liquid refrigerant is withdrawn and routed to the multiple expansion valves and evaporators.
- Various types of compressors may be used. For example, centrifugal, reciprocating, rotary screw and scroll compressors. Each application favors one or another type due to size, noise, efficiency and pressure parameters.
- Some refrigeration units may have multiple compressor stages which requires multiple compressors in various arrangements.[4]
Other items of interest are:
- More details about vapor-compression refrigeration systems are available in the classic "Perry's Chemical Engineers' Handbook".[5]
- The cooling capacity of refrigeration systems is often defined in units called "tons of refrigeration". The most common definition of that unit is: 1 ton of refrigeration is the rate of heat removal required to freeze a short ton (i.e., 2000 pounds) of water at 32 °F in 24 hours. Based on the heat of fusion for water being 144 Btu per pound, 1 ton of refrigeration = 12,000 Btu/h = 12,660 kJ/h = 3.517 kW. Most residential air conditioning units range in capacity from about 1 to 5 tons of refrigeration. A less common definition is: 1 tonne of refrigeration is the rate of heat removal required to freeze a metric ton (i.e., 1000 kg) of water at 0 °C in 24 hours. Based on the heat of fusion being 334.9 kJ/kg, 1 tonne of refrigeration = 13,954 kJ/h = 3.876 kW which is 10 percent larger than 1 ton of refrigeration.
History
In 1805, American inventor Oliver Evans described in detail, but never built, a refrigeration system based on the vapor-compression refrigeration cycle.[6]
An American living in Great Britain, Jacob Perkins, improved upon the design proposed by Oliver Evans and obtained the first patent for a vapor-compression refrigeration system in 1834.[7] Perkins built a prototype system and it actually worked.[8][9] According to the drawing in Perkins' patent, liquid ether (C4H10O) was contained in an "evaporator vessel" where it was vaporized under a partial vacuum maintained by the suction of a crude hand-operated compressor. The evaporator vessel was submerged in a liquid from which the heat required to vaporize the ether was extracted, thereby cooling the liquid. The compressed ether vapor from the compressor discharge was then condensed back into liquid either by flowing through piping coils submerged in water. The liquid ether then returned through a pressure reduction valve (i.e., an expansion valve) into the partial vacuum of the evaporator vessel. Clearly, Perkins' system included the four principal features uses by modern vapor-compression refrigeration systems, namely an evaporator, a compressor, a condenser and an expansion valve. Unfortunately, Perkins had no success in commercializing his system.[8]
In 1842, an American physician, John Gorrie, designed the first system for refrigerating water to produce ice. He also conceived the idea of using his refrigeration system to cool the air in the rooms of a Florida hospital used for treating yellow-fever and malaria patients. His system compressed air, then partially cooled the hot compressed air with water before allowing it to isentropically expand while doing part of the work required to drive the air compressor. The isentropic expansion cooled the air to a temperature low enough to freeze water and produce ice, or to flow "through a pipe for effecting refrigeration otherwise" as stated in his United States patent granted in 1851.[10] Gorrie, who had given up his medical practice, built a working prototype and sought to raise money to manufacture his machine, but the venture failed and his system was a commercial failure.
Alexander Catlin Twining,[11] a professor of engineering, mathematics and astronomy at Middlebury College in Connecticut, began experimenting with vapor-compression refrigeration in 1848 and obtained British and American patents in 1850 and 1853 for a vapor-compression system capable of using either carbon dioxide (CO2), ammonia (NH3) or ether.[12] He is credited by many with having initiated commercial refrigeration in the United States by building an ice plant in 1855 at Cleveland, Ohio that produced about 2000 pounds (900 kilograms) of ice per 24 hours.
Meanwhile in Australia, engineer James Harrison[13] built a commercial ice-making machine in 1854 and his patent for a vapor-compression refrigeration system using liquid ether was granted in 1855. Harrison introduced commercial vapor-compression refrigeration to breweries and meat packing houses, and by 1861 a dozen of his systems were in operation.
Carl von Linde[14], an engineering professor at the Technological University Munich in Germany, patented an improved method of liquefying gases in 1876. His new process made possible using gases such as ammonia, sulfur dioxide (SO2) and methyl chloride (CH3Cl) as refrigerants and they were widely used for that purpose until the late 1920’s. By then, a number of accidents related to the use of those refrigerants convinced manufacturers that a more stable element was needed. That led to the development and widespread use of chlorofluorocarbons (CFCs) until it was found that CFCs led to damaging of the Earth's ozone layer and their use was largely phased out in favor of refrigerants that do not contain any chlorine (Cl).
References
- ↑ Jump up to: 1.0 1.1 Yunus A. Çengal and Robert H. Turner (2004). Fundamentals of Thermal-Fluid Sciences, 2nd Edition. McGraw-Hill Professional. ISBN 0-07-245426-1.
- ↑ The Ideal Vapor-Compression Cycle
- ↑ Jump up to: 3.0 3.1 3.2 Y.V.C. Rao (2004). An Introduction to Thermodynamics, 2nd Edition. Universities Press (India). ISBN 81-7371-461-4.
- ↑ Schematic diagrams of multi-stage units
- ↑ Perry, R.H. and Green, D.W. (1984). Perry's Chemical Engineers' Handbook, 6th Edition. McGraw Hill, Inc.. ISBN ISBN 0-07-049479-7. (see pages 12-27 through 12-38)
- ↑ Oliver Evans (1805). The Abortion of the Young Engineer's Guide. Fry and Kammerer, Philadelphia. Available online at History Department, University of Rochester See the last item in the Appendix
- ↑ British patent 6662. August, 1834.
- ↑ Jump up to: 8.0 8.1 Norman Selfe (1900). Machinery for Refrigeration. H.S. Rich & Co. (Press of Ice and Refrigeration, Chicago), pp 18-19. Full copy available at Google books
- ↑ Aubrey F. Burstall (1965). A History of Mechanical Engineering. The MIT Press. ISBN 0-262-52001-X.
- ↑ "Improved process for the artificial production of ice", U.S. Patent Office, Patent 8080, 1851
- ↑ Alexander Catlin Twining, LL.D
- ↑ British patent 13,167 in 1850 and U.S. patent 10,221 in 1853
- ↑ Harrison, James]
- ↑ Von Linde, Carl