Arcuate nucleus: Difference between revisions
imported>Gareth Leng (live) |
imported>Gareth Leng |
||
| (26 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{subpages}} | ||
{{Image|Median eminence.jpg|right|450px| Schematic view of the ventral surface of the brain, at the level of the hypothalamus. The median eminence is at the bottom, and receives projections from the neuroendocrine neurons of the arcuate nucleus}} | |||
The '''arcuate nucleus''' is a small part of the hypothalamus that plays an extremely important role in the regulation of hormone secretion from the pituitary gland, and in the regulation of appetite and body weight. | |||
}} | |||
The | The arcuate nucleus is located in the mediobasal [[hypothalamus]] at the base of the brain, it lies on either side of the [[third ventricle]] and just above the [[median eminence]]. It includes several important populations of neurons, including neuroendocrine neurones, and appetite-regulating neurones. | ||
==Neuroendocrine Neurons== | |||
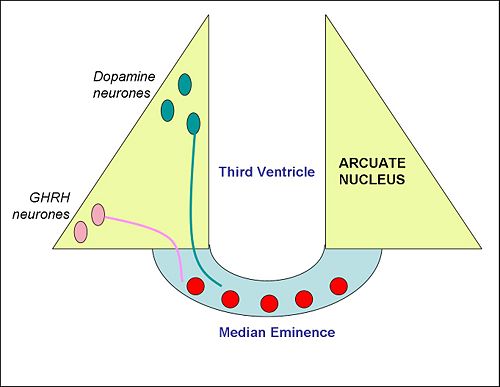
{{Image|Arcuate nucleus.jpg|right|500px| Schematic cross section of the mediobasal hypothalamus, with the median eminence at the bottom, showing the neuroendocrine neurons of the arcuate nucleus}} | |||
The arcuate nucleus contains two important populations of [[Neuroendocrinology |neuroendocrine neurons]] with nerve endings in the median eminence. One population releases [[dopamine]] into the hypophysial portal blood to regulate the secretion of the hormone [[prolactin]], which in turn controls the production of milk ([[lactogenesis]]). These are sometimes called the "tuberoinfundibular dopamine" (TIDA) neurons. In lactating females, TIDA neurons are inhibited by the stimulus of suckling. Dopamine released from their nerve endings at the median eminence is transported to the [[anterior pituitary gland]], where it inhibits prolactin secretion, so, when the TIDA neurons are inhibited, prolactin secretion is increased. Dopamine neurons of the arcuate also inhibit the release of [[gonadotropin-releasing hormone]], explaining in part why lactating (or otherwise [[hyperprolactinemia|hyperprolactinemic]]) women experience oligomenorrhea or amenorrhea (infrequency or absence of menses).<ref>Voogt JL ''et al'' (2001) Regulation of prolactin secretion during pregnancy and lactation. ''Prog Brain Res'' '''133''':173-85 PMID 11589129</ref> | |||
A second population of neuroendocrine neurons, mainly in the ventrolateral part of the arcuate nucleus, make [[growth hormone-releasing hormone]] (GHRH). Like the TIDA neurons, these neurons have nerve endings in the median eminence. GHRH released into the hypophysial portal blood is transported to the anterior pituitary gland, where it stimulates the secretion of [[growth hormone]]. <ref>Bluet-Pajot MT ''et al'' (1998) Hypothalamic and hypophyseal regulation of growth hormone secretion. ''Cell Mol Neurobiol'' 18:101-23 PMID 9524732</ref>. | |||
==Appetite-regulating neurones== | |||
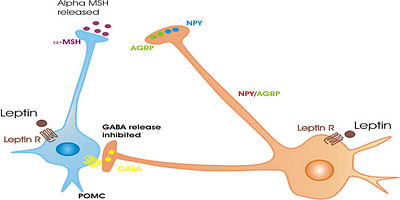
{{Image|LeptinactiononPOMCneurones.jpg|left|400px|}} | |||
The arcuate nucleus has a key role in the regulation of feeding behaviour, through two important populations of centrally projecting neurons: | |||
* | * Neurons that contain [[neuropeptide Y]] (NPY); another peptide, [[agouti-related protein]] (AGRP); and the inhibitory neurotransmitter [[GABA]]. These neurons, in the most ventromedial part of the nucleus, project strongly to the [[lateral hypothalamus]] and to the [[paraventricular nucleus]] of the hypothalamus, and are important in the regulation of [[appetite]]. NPY and AGRP are both very potent stimulators of feeding behaviour. The expression of NPY and AgRP is strongly increased after a period of fasting, and when activated, these neurons can produce ravenous eating when food is available. These neurons are regulated by circulating concentrations of [[leptin]] (a hormone secreted from fat cells ([[adipose tissue]]) and [[ghrelin]] (a hormone secretd from the stomach when it is empty). Destruction of these neurons leads to severe loss of appetite in experimental animals <ref>Sawchenko PE (1998) Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. ''J Comp Neurol'' 402:435-41 PMID 9862319</ref><ref>Bouret SG, Simerly RB (2006) Developmental programming of hypothalamic feeding circuits. ''Clin Genet'' 70:295-301 PMID 16965320</ref><ref> | ||
van den Top M, Spanswick D (2006) Integration of metabolic stimuli in the hypothalamic arcuate nucleus. ''Prog Brain Res'' 153:141-54 PMID 16876573</ref> | |||
* | * Neurons that contain [[peptide]] products of [[pro-opiomelanocortin]] (POMC), notably including the appetite suppressing peptide [[alpha-melanocyte stimulating hormone]] (alpha-MSH) and the [[opioid]] peptide [[beta-endorphin]]; they also express another appetite-inhibiting peptide, [[cocaine-and-amphetamine-regulating transcript]] (CART), and some make the neurotransmitter [[acetylcholine]]. These neurons project to many brain areas, including to all parts of the hypothalamus. They are also very important in the regulation of [[appetite]], and, when activated, they inhibit feeding, mainly via the effects of alpha MSH. These neurons are also regulated by circulating concentrations of [[leptin]] and [[ghrelin]]. They are directly innervated by the NPY/AGRP neurons, which inhibit them mainly via the release of GABA. POMC neurons that project to the medial preoptic nucleus are also involved in the regulation of [[sexual behavior]] in both males and females. <ref>Cone RD ''et al.'' (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. ''Int J Obes Relat Metab Disord'' 25 Suppl 5:S63-7 PMID 11840218</ref><ref>Cone RD (2005) Anatomy and regulation of the central melanocortin system. ''Nat Neurosci'' 8:571-8 PMID 15856065</ref> | ||
The arcuate nucleus also contains a population of specialized [[astrocytes]], called [[tanycytes]]. | ==Other neuronal cell types== | ||
The arcuate nucleus contains some other neuronal cell types the function of which is still not fully known. In particular, it contains: | |||
* Centrally-projecting neurons that make somatostatin; the neurosecretory somatostatin neurons that regulate growth hormone secretion are a different population, located in the periventricular nucleus.<ref>{{cite journal |author=Kawano H, Daikoku S |title=Somatostatin-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies|journal=J Comp Neurol |volume=271 |pages=293–9 |year=1988 |pmid=2897982}}</ref> | |||
* A small population of neurons that make the potent appetite-stimulating peptide [[ghrelin]]. The role of this population is not known; many neurons in the arcuate nucleus have receptors for ghrelin, but these are thought to respond mainly to blood-borne ghrelin, secreted by the empty stomach.<ref>Elmquist JK ''et al.'' (2005) Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis ''J Comp Neurol'' 493:63-71 PMID 16254991</ref> | |||
* Neurons that synthesise [[kisspeptin]], a peptide that is a major regulator of the secretion of luteinizing hormone releasing hormone (LHRH). These neurones co-express the opi0d peptide [[dynorphin]] and neurokinin, and appear to be specifically involved in the regulation of pulsatile LHRH release. | |||
The arcuate nucleus also contains a population of specialized [[astrocytes]], called [[tanycytes]]. These are thought to be actively involved in regulating neuroendocrine hormone secretion from neurosecretory nerve terminals. | |||
==Blood-brain barrier== | |||
Because the appetite regulating neurones of the arcuate nucleus respond directly to blood-borne ghrelin and laptin, it is generally assumed that these peptides must cross the blood-brain barrier at the arcuate nucleus. In neonatal rats, the blood-brain barrier is certainly permeable in this region, but in adult animals this is more controversial. The nucleus is adjacent to the median eminence, the external zone of which has fenestrated capillaries and is clearly outside the blood-brain barrier, but the internal zone of the median eminence appears to be protected by the barrier. | |||
==References== | ==References== | ||
{{reflist | 2}} | |||
{{ | |||
Revision as of 09:43, 5 August 2011
The arcuate nucleus is a small part of the hypothalamus that plays an extremely important role in the regulation of hormone secretion from the pituitary gland, and in the regulation of appetite and body weight.
The arcuate nucleus is located in the mediobasal hypothalamus at the base of the brain, it lies on either side of the third ventricle and just above the median eminence. It includes several important populations of neurons, including neuroendocrine neurones, and appetite-regulating neurones.
Neuroendocrine Neurons
The arcuate nucleus contains two important populations of neuroendocrine neurons with nerve endings in the median eminence. One population releases dopamine into the hypophysial portal blood to regulate the secretion of the hormone prolactin, which in turn controls the production of milk (lactogenesis). These are sometimes called the "tuberoinfundibular dopamine" (TIDA) neurons. In lactating females, TIDA neurons are inhibited by the stimulus of suckling. Dopamine released from their nerve endings at the median eminence is transported to the anterior pituitary gland, where it inhibits prolactin secretion, so, when the TIDA neurons are inhibited, prolactin secretion is increased. Dopamine neurons of the arcuate also inhibit the release of gonadotropin-releasing hormone, explaining in part why lactating (or otherwise hyperprolactinemic) women experience oligomenorrhea or amenorrhea (infrequency or absence of menses).[1]
A second population of neuroendocrine neurons, mainly in the ventrolateral part of the arcuate nucleus, make growth hormone-releasing hormone (GHRH). Like the TIDA neurons, these neurons have nerve endings in the median eminence. GHRH released into the hypophysial portal blood is transported to the anterior pituitary gland, where it stimulates the secretion of growth hormone. [2].
Appetite-regulating neurones
The arcuate nucleus has a key role in the regulation of feeding behaviour, through two important populations of centrally projecting neurons:
- Neurons that contain neuropeptide Y (NPY); another peptide, agouti-related protein (AGRP); and the inhibitory neurotransmitter GABA. These neurons, in the most ventromedial part of the nucleus, project strongly to the lateral hypothalamus and to the paraventricular nucleus of the hypothalamus, and are important in the regulation of appetite. NPY and AGRP are both very potent stimulators of feeding behaviour. The expression of NPY and AgRP is strongly increased after a period of fasting, and when activated, these neurons can produce ravenous eating when food is available. These neurons are regulated by circulating concentrations of leptin (a hormone secreted from fat cells (adipose tissue) and ghrelin (a hormone secretd from the stomach when it is empty). Destruction of these neurons leads to severe loss of appetite in experimental animals [3][4][5]
- Neurons that contain peptide products of pro-opiomelanocortin (POMC), notably including the appetite suppressing peptide alpha-melanocyte stimulating hormone (alpha-MSH) and the opioid peptide beta-endorphin; they also express another appetite-inhibiting peptide, cocaine-and-amphetamine-regulating transcript (CART), and some make the neurotransmitter acetylcholine. These neurons project to many brain areas, including to all parts of the hypothalamus. They are also very important in the regulation of appetite, and, when activated, they inhibit feeding, mainly via the effects of alpha MSH. These neurons are also regulated by circulating concentrations of leptin and ghrelin. They are directly innervated by the NPY/AGRP neurons, which inhibit them mainly via the release of GABA. POMC neurons that project to the medial preoptic nucleus are also involved in the regulation of sexual behavior in both males and females. [6][7]
Other neuronal cell types
The arcuate nucleus contains some other neuronal cell types the function of which is still not fully known. In particular, it contains:
- Centrally-projecting neurons that make somatostatin; the neurosecretory somatostatin neurons that regulate growth hormone secretion are a different population, located in the periventricular nucleus.[8]
- A small population of neurons that make the potent appetite-stimulating peptide ghrelin. The role of this population is not known; many neurons in the arcuate nucleus have receptors for ghrelin, but these are thought to respond mainly to blood-borne ghrelin, secreted by the empty stomach.[9]
- Neurons that synthesise kisspeptin, a peptide that is a major regulator of the secretion of luteinizing hormone releasing hormone (LHRH). These neurones co-express the opi0d peptide dynorphin and neurokinin, and appear to be specifically involved in the regulation of pulsatile LHRH release.
The arcuate nucleus also contains a population of specialized astrocytes, called tanycytes. These are thought to be actively involved in regulating neuroendocrine hormone secretion from neurosecretory nerve terminals.
Blood-brain barrier
Because the appetite regulating neurones of the arcuate nucleus respond directly to blood-borne ghrelin and laptin, it is generally assumed that these peptides must cross the blood-brain barrier at the arcuate nucleus. In neonatal rats, the blood-brain barrier is certainly permeable in this region, but in adult animals this is more controversial. The nucleus is adjacent to the median eminence, the external zone of which has fenestrated capillaries and is clearly outside the blood-brain barrier, but the internal zone of the median eminence appears to be protected by the barrier.
References
- ↑ Voogt JL et al (2001) Regulation of prolactin secretion during pregnancy and lactation. Prog Brain Res 133:173-85 PMID 11589129
- ↑ Bluet-Pajot MT et al (1998) Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol Neurobiol 18:101-23 PMID 9524732
- ↑ Sawchenko PE (1998) Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402:435-41 PMID 9862319
- ↑ Bouret SG, Simerly RB (2006) Developmental programming of hypothalamic feeding circuits. Clin Genet 70:295-301 PMID 16965320
- ↑ van den Top M, Spanswick D (2006) Integration of metabolic stimuli in the hypothalamic arcuate nucleus. Prog Brain Res 153:141-54 PMID 16876573
- ↑ Cone RD et al. (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25 Suppl 5:S63-7 PMID 11840218
- ↑ Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571-8 PMID 15856065
- ↑ Kawano H, Daikoku S (1988). "Somatostatin-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies". J Comp Neurol 271: 293–9. PMID 2897982.
- ↑ Elmquist JK et al. (2005) Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis J Comp Neurol 493:63-71 PMID 16254991