Zidovudine: Difference between revisions
imported>David E. Volk (New page: {{subpages}} {{Chem infobox |align=right |image=right|thumb|200px |width=200px |molname=zidovudine |synonyms= see below |molformula= C<sub>10</sub>H<sub>13</su...) |
imported>David E. Volk |
||
| Line 20: | Line 20: | ||
== Mechanism of action == | == Mechanism of action == | ||
Zidovudine is a nucleoside-based reverse transcriptase | Zidovudine is a nucleoside-based [[reverse transcriptase]] [[reverse transcriptase inhibitor|inhibitor]] that is structurally analogous to [[thymidine]], a normal base in DNA. After phosphorylation, it becomes incorporated into viral DNA where it acts as a DNA chain terminator. The viral DNA chain is terminated because the 3'-hydroxy group needed for the formation of a phosphordiester bond is missing, having been replaced by an azido group. It also inhibits the HIV RT by binding with it and thus competing with natural substrates. | ||
== Chemistry == | == Chemistry == | ||
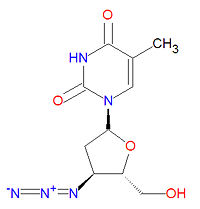
The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C<sub>10</sub>H<sub>13</sub>N<sub>5</sub>O<sub>4</sub>, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA. | The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C<sub>10</sub>H<sub>13</sub>N<sub>5</sub>O<sub>4</sub>, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA. | ||
Revision as of 17:35, 28 March 2008
|
| |||||||
| zidovudine | |||||||
| |||||||
| Uses: | antiviral drug HIV | ||||||
| Properties: | reverse transcriptase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Zidovudine is an antiviral drug used primarily for the treatment of HIV/AIDS.
Mechanism of action
Zidovudine is a nucleoside-based reverse transcriptase inhibitor that is structurally analogous to thymidine, a normal base in DNA. After phosphorylation, it becomes incorporated into viral DNA where it acts as a DNA chain terminator. The viral DNA chain is terminated because the 3'-hydroxy group needed for the formation of a phosphordiester bond is missing, having been replaced by an azido group. It also inhibits the HIV RT by binding with it and thus competing with natural substrates.
Chemistry
The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C10H13N5O4, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA.