Proline: Difference between revisions
imported>David E. Volk (stub and structure) |
imported>Caesar Schinas m (Bot: Update image code) |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
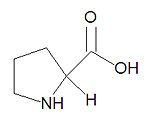
{{Image|Proline stick figure.jpg|right|150px|'''Proline''', a common amino acid.}} | |||
'''Proline''', abbreviated '''Pro''' or '''P''', is one of the twenty common <math>\alpha</math>-[[amino acid]]s used by living organisms to build [[protein]]s. It is the only cyclic amino acid and because of its restricted allowed conformations, it often ends [[secondary structure]] elements in proteins and forms turns in protein structures. It is aliphatic, cyclic and nonpolar. It is the only amino acid that does not have an amide proton. | '''Proline''', abbreviated '''Pro''' or '''P''', is one of the twenty common <math>\alpha</math>-[[amino acid]]s used by living organisms to build [[protein]]s. It is the only cyclic amino acid and because of its restricted allowed conformations, it often ends [[secondary structure]] elements in proteins and forms turns in protein structures. It is aliphatic, cyclic and nonpolar. It is the only amino acid that does not have an amide proton. ''Technically'', because the proline side chain bonds to both the alpha carbon and the backbone nitrogen atom, the nitrogen is a secondary amine, making proline an ''imino acid'' rather than an ''amino acid''. However, it is typically referred to as an amino acid. | ||
== Biosynthesis == | |||
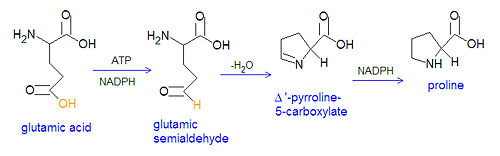
The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an [[acyl phosphate]] by reacting with ATP, and the acyl phosphate is reduced to [[glutamatic-γ-semialdehyde]] by [[NADPH]]. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to form Δ'-[[Pyrroline-5-carboxylate]], which can be reduced by NADPH to form proline. | |||
{{Image|Proline synthesis.jpg|center|500px|Biosynthesis of proline via glutamate semialdehyde and pyrroline. Note that the neutral froms of the chemicals are shown, rather than deprotonated acid groups and protonated amino groups.}} | |||
Latest revision as of 08:46, 8 June 2009
Proline, abbreviated Pro or P, is one of the twenty common -amino acids used by living organisms to build proteins. It is the only cyclic amino acid and because of its restricted allowed conformations, it often ends secondary structure elements in proteins and forms turns in protein structures. It is aliphatic, cyclic and nonpolar. It is the only amino acid that does not have an amide proton. Technically, because the proline side chain bonds to both the alpha carbon and the backbone nitrogen atom, the nitrogen is a secondary amine, making proline an imino acid rather than an amino acid. However, it is typically referred to as an amino acid.
Biosynthesis
The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an acyl phosphate by reacting with ATP, and the acyl phosphate is reduced to glutamatic-γ-semialdehyde by NADPH. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to form Δ'-Pyrroline-5-carboxylate, which can be reduced by NADPH to form proline.