Health consequences of obesity: Difference between revisions
imported>Gareth Leng |
imported>Gareth Leng |
||
| (44 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Obesity final.jpg|right|450px|(Adapted from <ref>Boon NA ''et al.'' (2006) ''Davidson's Principles and Practice of Medicine'' p 111</ref>)}} | |||
{{Image|Obesity final.jpg|right| | [[Obesity]] is conveniently defined according to [[Body Mass Index]] (BMI) such that a BMI in excess of 30kg/m<sup>2</sup> categorises someone as obese; the main '''health consequences of obesity''' are summarised below. This article focusses on the cardiovascular consequences, [[nonalcoholic fatty liver disease]] (NAFLD), endocrine changes and psychosocial consequences of obesity as these are generally identified as the most significant complications. The implication of the association between [[type 2 diabetes mellitus]] and obesity is discussed in [[Diabesity]]. | ||
This article | |||
===Cardiovascular Disease in Obesity=== | ===Cardiovascular Disease in Obesity=== | ||
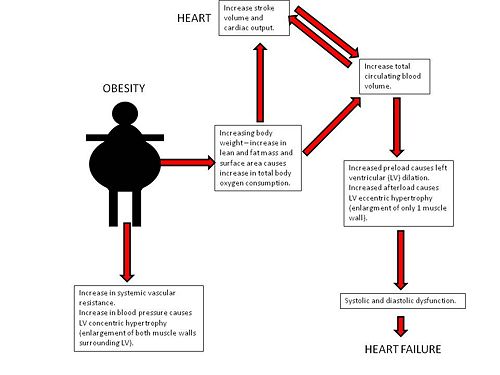
Obesity is associated with many serious diseases, including[[coronary heart disease]] (CHD) | Obesity is associated with many serious diseases, including [[coronary heart disease]] (CHD), and BMI and waist circumference are positively correlated with measures of risk for CHD such as [[hypertension]] and blood lipid concentrations<ref name=Kopelman2000> Kopelman PG (2000) Obesity as a medical problem ''Nature'' 404:635-42 PMID 10766250</ref>. Obesity increases the risk of cardiovascular disease and premature death, and this may be indirectly mediated through risk factors associated with the [[metabolic syndrome]]. Central deposition of [[adipose tissue]] increases the risk of cardiovascular morbidity and mortality, including [[stroke]], [[congestive heart failure]], [[myocardial infarction]] and cardiovascular death<ref name=Gaal2006>Gaal LFV ''et al.''(2006) Mechanisms linking obesity with cardiovascular disease ''Nature'' 444:875-9</ref>. Waist-hip ratios are commonly used to assess this type of body fat distribution. | ||
Obesity | Obesity causes an increase in [[total body oxygen consumption]] due to excess lean tissue mass as well as the oxidative demands of metabolically active adipose tissue, resulting in an increase in [[cardiac output]]. As a result, the [[left ventricle]] dilates to accommodate the increased venous return with subsequent development of eccentric hypertrophy to keep the wall stress normal<ref name=Mathew2008>Mathew B ''et al.'' {2008) Obesity: effects on cardiovascular disease and its diagnosis ''J Am Board Fam Med'' 21:562-8 PMID 18988724</ref>. Eventually, the ventricle can no longer adapt to volume overload, resulting in decreased ventricular contractility. With left ventricular hypertrophy, reduced ventricular compliance alters the ability of the chamber to accommodate an increased volume during [[diastole]], and this results in diastolic dysfunction. A combination of systolic and diastolic dysfunction leads to heart failure. | ||
{{Image|slide1.jpg|right|500px|}} | |||
[[Hypertension]] also becomes more prevalent with increasing obesity. In men, a BMI of <25 or >30 shows a prevalence of hypertension of 15% and 42%, respectively; in women, 15% and 38%, respectively. [[Arrhythmia]]s may be the most frequent cause of death among obese people, as increased catecholamine and free fatty acid levels may affect repolarization. The Framingham Study shows that sudden cardiac death was 40 times higher in obese men and women. In the NHANES III study, 30% of obese patients with glucose intolerance had a prolonged corrected QT (QTc) interval. A QTc of >0.42s was associated with increased mortality in “healthy” obese patients Schouten ''et al.'' found that 8% of obese individuals had QTc interval of >0.44s and in 2% it was >0.46s. | |||
Increased adiposity and reduced physical activity are strong and independent predictors of CHD and death: for each unit increase in BMI, the risk of CHD increases by 8%. However, each 1 hour metabolic equivalent increase in activity score decreases CHD risk by 8%. Physical activity increases myocardial oxygen supply, improving myocardial contraction and electrical stability. <ref name=Gaal2006> Obesity is an independent predictor of [[coronary artery disease]], and this is also linked to BMI; obesity accelerates [[atherosclerosis]] many years before the clinical signs become obvious. In autopsies among 15-35 year olds who died from accidental causes, plaques and ulceration in the coronary arteries and [[abdominal aorta]] were found and the extent of damage was related to the amount of abdominal fat and BMI.<ref name=Mathew2008 /> | |||
The risk of [[stroke]]increases with increased BMI and waist-hip ratio. In the prospective Physician’s Health Study, results showed that an increase of 1 BMI unit, increased the rate of ischemic stroke by 4% and haemorrhagic stroke by 6%. The underlying mechanisms linking increased BMI to increased stroke risk are not clear but it is thought that they could involve the prothrombotic and proinflammatory state in obesity. Adipose tissue is as an active endocrine organ: release of [[adipokine]]s (including [[leptin]] and [[adiponectin]]), proinflammatory cytokines (IL-6 and CRP) and hypofibrinolytic factors (PAI-1) might, together, lead to increased oxidative stress and endothelial dysfunction, promoting atherosclerosis which then leads to [[stroke]].<ref name=Gaal2006 /> Terao ''et al.'' (2008) investigated the effect of inflammatory and injury reposonse to ischaemic stroke in obese mice; when the middle cerebral artery was occluded and reperfused, the inflammatory and injury responses were worse in genetically obese mice (''ob/ob'') than in wild-type mice<ref name=Terao2008>Terao S ''et al.'' (2008) Inflammatory and injury responses to ischemic stroke in obese mice ''Stroke'' 39:943-50</ref>. Monoctye chemoattractant protein-1 appears to be involved in the exaggerated responses to ischaemic stroke in obese mice. | |||
== Non-alcoholic liver disease and obesity == | == Non-alcoholic liver disease and obesity == | ||
Nonalcoholic fatty liver disease | [[Nonalcoholic fatty liver disease]] is an accumulation of fat (mainly [[triglyceride]]s) in the hepatocytes that exceeds 5% of the liver weight, a condition known as ''[[steatosis]].'' If untreated, steatosis can lead to [[steatohepatitis]], which can result in [[fibrosis]], [[cirrhosis]] and liver failure. Patients with NAFLD complain of fatigue, malaise and feelings of discomfort or “fullness” in the upper right abdomen. Laboratory tests reveal a mild to moderate increase in serum levels of [[alanine aminotransferase]], [[aspartate aminotransferase]] or both.<ref name=angulo2002>Angulo P (2002) Nonalcoholic fatty liver disease ''New Eng J Med'' 346:1221-3</ref> The ratio of aspartate aminotransferase to alanine aminotransferase is usually less than 1, but as fibrosis in the liver advances, this ratio increases. NAFLD affects 10 -24% of the world’s population, and 58-74% of obese persons, including 2.6% of children and 23-53% of obese children.<ref name=Angulo2007>Angulo P (2007) Obesity and nonalcoholic fatty liver disease, ''Nutr Rev'' 65:s57-s63</ref>. | ||
Patients | |||
'''Risk Factors:''' | '''Risk Factors:''' | ||
Insulin resistance and | Insulin resistance and NAFLD are commonly associated with the central obesity phenotype, as visceral fat has a greater lipolytic potential than subcutaneous fat. The fatty acids which are released from visceral fat during lipolysis drain straight into the portal circulation. It is the increased lecels of free fatty acids in the circulation which are thought to be responsible for insulin resistance.<ref name=stranges2004>Stranges S ''et al.''(2004) Body fat distribution, relative weight, and liver enzyme levels: a population based study ''Hepatology'' 39:754-63</ref> | ||
'''The role of Insulin Resistance in NAFLD:''' | '''The role of Insulin Resistance in NAFLD:''' | ||
[[Insulin resistance]] | [[Insulin resistance]] is thought to be the leading cause of NAFLD. It is thought that fat accumulation in the hepatocytes occurs via [[lipolysis]] and [[hyperinsulinaemia]]. Microsomal ω-oxidation has been found to produce clinically significant amounts of cytotoxic dicarboxylic acids. This pathway of fatty acid metabolism is closely related to mitochondrial β-oxidation and peroxisomal β-oxidation. A lack in the enzymes associated with peroxisomal β-has been identified as a major cause of [[steatosis]] and [[steatohepatitis]]. An example of this is deficiency of [[acyl-coenzyme A oxidase]] which disrupts the oxidation of long fatty acid chains and dicarboxilic acids, leading to microvesicular steatosis and steatohepatitis. Loss of function of acyl-coenzyme A oxidase also causes sustained hyperactivation of [[peroxisome proliferator activated receptor-α]] (PPAR- α), leading to upregulation of PPAR-α regulated genes <ref name=Fan1998>Fan C''et al.''(1998) Steatohepatitis, spontaneousperoxisome proliferation and liver tumours in mice lacking fatty acyl CoA ocidase; implications for peroxisome proliferator activated receptor alpha ligand metabolism ''J Biol Chem'' 273:15639-45</ref>. Studies suggest that PPAR-α is responsible for promoting the synthesis of [[uncoupling protein]] 2 in the liver <ref name=Chavin1999>Chavin KD ''et al.'' (1999) Obesity increases expression of uncoupling protein 2 in hepatocytes and promotes liver ATP depletion. ''J Biol Chem'' 274:5692-700</ref>. Increased levels of fatty acids in the liver provide a source of oxidative stress, which is thought to be responsible for the progression from steatosis to steatohepatitis and finally to cirrhosis. | ||
Microsomal ω-oxidation has been found to produce clinically significant amounts of cytotoxic | |||
'''The role of the keratin cytosleleton | '''The role of the keratin cytosleleton''' | ||
Mature and differentiated hepatocytes are the epithelial cells of the liver and normally express | Mature and differentiated [[hepatocytes]] are the epithelial cells of the [[liver]] and normally express type I [[keratin]] (keratin 8) and type II keratin (keratin 18), arranged into [[intermediate filament]]s in the [[cytoplasm]]. In NAFLD, the keratin cytoskeleton in hepatocytes is disrupted, <ref name=Zatoukal2003>Zatloukal K (2003) The keratin cytoskeleton in liver diseases ''J Pathol'' 204:367-76</ref> apparently due to [[oxidative stress]].<ref name=Zatoukal2003 /> | ||
==Endocrine Changes in Obesity== | ==Endocrine Changes in Obesity== | ||
Obesity is associated with changes in the normal endocrine profile | Obesity is associated with changes in the normal endocrine profile, particularly in the sex steroid profile. | ||
'''Oestrogens:''' | '''Oestrogens:''' [[Oestrogen]]s are synthesized by aromatization of circulating [[testosterone]]s, catalysed by the enzyme [[aromatase]]. This occurs at many sites throughout the body, including in adipose tissue <ref name=Purohit2002>Purohit A, Reed MJ (2002) Regulation of oestrogen synthesis in postmenopausal women. ''Steroids'' 67:979-83</ref> <ref name=Bianchini2002>Bianchini F ''et al.'' (2002) Overweight, obesity, and cancer risk. ''Lancet Oncol'' 3:565-74</ref>. An increase in adipose tissue mass results in a greater capacity for aromatization, and an increase in oestrogen levels <ref name=Bates1982>Bates GW, Whitworth N (1982) Effects of obesity on sex steroid metabolism. ''J Chronic Dis'' 35:893</ref>. | ||
In premenopausal, non-pregnant women, the principal site of aromatization is the ovaries and only a minor proportion of oestrogen comes from adipose tissue. However, in postmenoapusal women, peripheral aromatisation is enhanced and adipose tissue becomes the main site of oestrogen production <ref name=Simpson2000>Simpson ER (2000) Role of aromatase in sex steroid action. ''J Mol Endocrinol'' 25:149-56</ref>. | In premenopausal, non-pregnant women, the principal site of aromatization is the ovaries, and only a minor proportion of oestrogen comes from adipose tissue. However, in postmenoapusal women, peripheral aromatisation is enhanced and adipose tissue becomes the main site of oestrogen production <ref name=Simpson2000>Simpson ER (2000) Role of aromatase in sex steroid action. ''J Mol Endocrinol'' 25:149-56</ref>. | ||
'''Androgens''': The increased capacity for aromatization results in hypoandrogenism in males, as | '''Androgens''': The increased capacity for aromatization results in hypoandrogenism in males, as more circulating testosterone is converted to oestrogen <ref name=Hammoud>Hammoud AO ''et al.'' Obesity and male reproductive potential ''J Androl'' 27:619–25</ref>. Other factors contributing to the decrease in circulating testosterone include insulin resistance and the suppression of the hypothalamo-pituitary-testicular axis. In contrast, in obese premenopausal women, obesity is associated with an increase in free testosterone levels <ref name=Lukanova2004>Lukanova A ''et al.'' (2004) Body mass index, circulating levels of sex steroid hormones, IGF-1 and IGF-binding protein-3: a cross-sectional study in healthy women. ''Eur J Endocrinol'' 190:161-71</ref> This is thought to be partly mediated by the increased levels of insulin and IGF-1 associated with obesity.<ref name=Metwally2007>Metwally M ''et al.''(2007) The impact of obesity on female reproductive function ''Obesity Rev''' 8:515-23</ref> Furthermore, as for oestrogen, adipose tissue is an important site of testosterone production, due to local expression of 17 beta hydroxysteroid dehydrogenase. Therefore there is a positive association between adipose tissue mass and androgen concentration. | ||
'''SHBG:'''Sex steroids are highly lipophilic and are | '''SHBG:''' Sex steroids are highly lipophilic and are carried in the circulation bound to [[sex hormone binding globulin]]s (SHBG.) Obesity results in a decreased concentration of SHBG <ref name=Haslam2005>Haslam DW, James WP (2005) Obesity ''Lancet'' 366:1197-209</ref>. This is thought to be associated with the elevated insulin levels associated with obesity, as insulin inhibits hepatic synthesis of SHBG. The decreased concentration of binding protein results in an increase in the free fraction of sex steroids <ref name= Lukanova2004 /> | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 67: | Line 55: | ||
| Decrease | | Decrease | ||
|} | |} | ||
'''Summary of the endocrine changes in obesity''' | |||
==Obesity and Cancer== | ==Obesity and Cancer== | ||
It is estimated that 10% of all [[cancer]] deaths among non-smokers are related to obesity <ref name=Haslam2005>Haslam DW, James WP (2005) Obesity ''Lancet'' 366:1197-209</ref>. It is hypothesised that alterations in hormone metabolism mediate the effects of obesity on cancer risk, due to sex steroid hormone regulation of cell proliferation, differentiation and [[apoptosis]]. Many types of cancer are more prevalent in obese subjects, the most widely studied of which are breast and [[endometrial cancer]]. These cancers are associated with an increase in [[oestrogen]] concentration, a decrease in plasma SHBG and an increase in [[androgen]] levels. <ref name=Bianchini2002>Bianchini F ''et al.'' (2002) Overweight, obesity, and cancer risk | It is estimated that 10% of all [[cancer]] deaths among non-smokers are related to obesity <ref name=Haslam2005>Haslam DW, James WP (2005) Obesity ''Lancet'' 366:1197-209</ref>. It is hypothesised that alterations in hormone metabolism mediate the effects of obesity on cancer risk, due to sex steroid hormone regulation of cell proliferation, differentiation and [[apoptosis]]. Many types of cancer are more prevalent in obese subjects, the most widely studied of which are breast and [[endometrial cancer]]. These cancers are associated with an increase in [[oestrogen]] concentration, a decrease in plasma SHBG and an increase in [[androgen]] levels. <ref name=Bianchini2002>Bianchini F ''et al.'' (2002) Overweight, obesity, and cancer risk ''Lancet Oncol'' 3:565-574</ref><ref name=Lukanova2004 /> These observations led to the ''unopposed oestrogen hypothesis'', which suggests that mitotic activity of cells is increased in the presence of oestrogen, unopposed by progestogens. <ref name=Key1988>Key TJ, Pike MC (1988) The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk ''B J Cancer'' 57:205-12</ref>. Increased mitotic activity leads to a higher prevalence of mutations, thus increasing the risk of cancer. | ||
==Obesity and Infertility== | ==Obesity and Infertility== | ||
Obesity is thought to account for around 6% of primary infertility <ref name=Haslam2005>Haslam, | Obesity is thought to account for around 6% of primary infertility <ref name=Haslam2005>Haslam DW, James WP (2005) Obesity ''Lancet'' 366:1197-209</ref>. | ||
''' | '''Women:''' Obese women are at increased risk of anovulatory infertility<ref name-Metwally2007>Metwally M ''et al.'' (2007) The impact of obesity on female reproductive function ''Obesity Rev'' 8:515-23</ref>. This is thought to be due to hyperandrogenism in which high androgen levels increase apoptosis of the [[granulosa cell]]s, as well as damaging the [[endometrium]] and developing [[oocyte]]s. Excess oestrogen also contributes to infertility by reducing [[gonadotrophin]] secretion through excess negative feedback <ref name=Hammoud>Hammoud AO ''et al.'' Obesity and male reproductive potential. ''J Androl'' 27:19–25</ref>. One of the commonest reproductive disorders in women is PCOS, which affects 5-10% of women of reproductive age <ref name=Strauss1999>Strauss JF, Dunaif A (1999) Molecular mysteries of polycystic ovary syndrome ''Mol Endocrinol'' 13;800-5</ref>. This syndrome is characterized by anovulatory infertility, obesity, [[hirsutism]], multiple [[ovarian cyst]]s and insulin resistance. Despite their being a well established association between obesity and PCOS, it remains unknown which is the cause and which is the effect. | ||
'''Men:''' "Hyperestrogenic hypogonadotropic hypogonadism" in obese men results in high oestrogen levels, low testosterone levels and subfertility. There is a negative association between [[spermatogenesis]] and increasing BMI. The mechanism mediating this association is yet to be identified, but it has been suggested that hypoandrogenism in obese males may result in a reduced production of [[testosterone]] by the [[testes]]. The hyperestrogenism may also cause inappropriate suppression of the hypothalamic-pituitary-gonadal axis, resulting in reduced [[spermatogenesis]]. Obesity has also been associated with [[erectile dysfunction]]. <ref name=Hammoud /> | |||
The relationship between obesity and mental health has been the subject of | ==='''Psychosocial Consequences of Obesity'''=== | ||
The relationship between obesity and mental health has been the subject of debate over the past 30 years. Early studies were consistent with the “jolly fat” hypothesis, suggesting that obesity confers a protective role against [[anxiety]] and depressive disorders, but more recent studies suggest that depression and obesity are linked. <ref name=Stunkard2003>Stunkard AJ ''et al.'' (2003) Depression and obesity ''Soc Biol Psychiat'' 54:330-7</ref> <ref name=Mather2009>Mather AA ''et al.'' (2009) Association of obesity with psychiatric disorders and suicidal behaviours in a nationally representative sample. ''J Psychosomatic Res''66:277-85</ref>. | |||
'''Recent hypotheses linking obesity and aggression:''' The underlying mechanisms and direction of an obesity-depression link remain largely unknown, although recent research has indicated that gender, obesity severity, comorbid physical illness, stress and abdominal fat distribution are important mediating risk factors for the development of an obesity-mental disorder link.<ref name= Rivenes2009/> <ref name= | '''Recent hypotheses linking obesity and aggression:''' The underlying mechanisms and direction of an obesity-depression link remain largely unknown, although recent research has indicated that gender, obesity severity, comorbid physical illness, stress and abdominal fat distribution are important mediating risk factors for the development of an obesity-mental disorder link.<ref name= Rivenes2009>Rivenes, AC ''et al.''(2009) The relationship between abdominal fat, obesity, and common mental disorders: results from the HUNT Study ''J Psychosomatic Res'' 66:269-75</ref> <ref name=Simon>Simon GE ''et al.'' (2008) Association between obesity and depression in middle-aged women ''Gen Hosp Psychiat'' 30:32-9</ref> <ref name=Scott2008>Scott KM ''et al.''(2008) Obesity and mental disorders in the general population: results from the World Mental Health Surveys ''Int J Obes'' 32:192-200</ref> These newly discovered mediators give rise to new hypotheses, involving over-activity of the [[hypothalamic-pituitary-adrenocortical]] axis. | ||
'''Experimental evidence:''' A | '''Experimental evidence:''' A nationally representative Canadian study found positive relationships between obesity and an array of lifetime psychiatric disorders and past-year mood and anxiety disorders. These <ref name=Stunkard2003/> <ref name=Scott2008/> <ref name=Simon /><ref name=Carpenter2000>Carpenter KM ''et al.''(2000) Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study ''Am J Pub Health'' 90:251–7</ref><ref name=Onyike2003>Onyike CU ''et al.''(2003) Is obesity associated with major depression? Results from the third National Health and utrition examination survey. ''Am J Epidemiol'' 158:1139–47</ref>. Subgroup analyses revealed that obese women were more susceptible to specific mental disorders than men, including [[depression]], mania, [[panic attack]]s, panic disorder, [[social phobia]] and [[agoraphobia]].<ref name=Mather2009/> These findings, which are consistent with other research, thus reinforce the notion that obese women are more vulnerable to developing mental disorders. This result may be explained in terms of the increased consciousness amongst women to conform to a socially desirable image and weight. The study also positively linked obesity to suicidal behaviours and negatively linked obesity with past-year drug dependence.<ref name=Dong2006>Dong C ''et al.''(2006) Extreme obesity is associated with suicide attempts: results from a family study ''Int J Obes'' 30:388-90</ref> The former may be attributed to the social stigmatization attached to obesity, inducing feelings of decreased self-worth and decreased self-esteem that fuel suicidal thoughts; and the latter due to protective effects of obesity, arising through food and addictive drugs competing for the same reward sites in the brain <ref name=Mather2009/><ref name=Kleiner2004>Kleiner KD ''et al.''(2004) Body mass index and alcohol use ''J Addict Dis'' 23:105-18</ref> | ||
'''Contradictory | '''Contradictory evidence:''' Some studies fail to identify mental disorders as a psychosocial consequence of obesity <ref name=Hasler2004>Hasler G ''et al.''(2004) The associations between psychopathology and being overweight: A 20-year prospective study ''Psychol Med'' 34:1047–157</ref><ref name=Faith2002>Faith MS ''et al.'' (2002) Obesity-depression associations in the population. ''J Psychosom Res'' 53:935–942</ref>. Similarly, whilst much research indicates sex differences between obesity and mental disorders, this is not a universal conclusion. In addition, some research only identifies associations amongst the severely obese, with a BMI of >35kg/m<small>2</small> <ref name=Scott2008/>. These variable conclusions may reflect methodological differences between studies. | ||
= | ==References== | ||
{{reflist | 2}} | |||
Latest revision as of 10:11, 24 July 2011
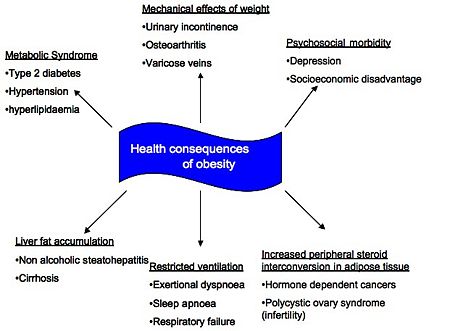
Obesity is conveniently defined according to Body Mass Index (BMI) such that a BMI in excess of 30kg/m2 categorises someone as obese; the main health consequences of obesity are summarised below. This article focusses on the cardiovascular consequences, nonalcoholic fatty liver disease (NAFLD), endocrine changes and psychosocial consequences of obesity as these are generally identified as the most significant complications. The implication of the association between type 2 diabetes mellitus and obesity is discussed in Diabesity.
Cardiovascular Disease in Obesity
Obesity is associated with many serious diseases, including coronary heart disease (CHD), and BMI and waist circumference are positively correlated with measures of risk for CHD such as hypertension and blood lipid concentrations[2]. Obesity increases the risk of cardiovascular disease and premature death, and this may be indirectly mediated through risk factors associated with the metabolic syndrome. Central deposition of adipose tissue increases the risk of cardiovascular morbidity and mortality, including stroke, congestive heart failure, myocardial infarction and cardiovascular death[3]. Waist-hip ratios are commonly used to assess this type of body fat distribution.
Obesity causes an increase in total body oxygen consumption due to excess lean tissue mass as well as the oxidative demands of metabolically active adipose tissue, resulting in an increase in cardiac output. As a result, the left ventricle dilates to accommodate the increased venous return with subsequent development of eccentric hypertrophy to keep the wall stress normal[4]. Eventually, the ventricle can no longer adapt to volume overload, resulting in decreased ventricular contractility. With left ventricular hypertrophy, reduced ventricular compliance alters the ability of the chamber to accommodate an increased volume during diastole, and this results in diastolic dysfunction. A combination of systolic and diastolic dysfunction leads to heart failure.
Hypertension also becomes more prevalent with increasing obesity. In men, a BMI of <25 or >30 shows a prevalence of hypertension of 15% and 42%, respectively; in women, 15% and 38%, respectively. Arrhythmias may be the most frequent cause of death among obese people, as increased catecholamine and free fatty acid levels may affect repolarization. The Framingham Study shows that sudden cardiac death was 40 times higher in obese men and women. In the NHANES III study, 30% of obese patients with glucose intolerance had a prolonged corrected QT (QTc) interval. A QTc of >0.42s was associated with increased mortality in “healthy” obese patients Schouten et al. found that 8% of obese individuals had QTc interval of >0.44s and in 2% it was >0.46s.
Increased adiposity and reduced physical activity are strong and independent predictors of CHD and death: for each unit increase in BMI, the risk of CHD increases by 8%. However, each 1 hour metabolic equivalent increase in activity score decreases CHD risk by 8%. Physical activity increases myocardial oxygen supply, improving myocardial contraction and electrical stability. Cite error: Closing </ref> missing for <ref> tag. Monoctye chemoattractant protein-1 appears to be involved in the exaggerated responses to ischaemic stroke in obese mice.
Non-alcoholic liver disease and obesity
Nonalcoholic fatty liver disease is an accumulation of fat (mainly triglycerides) in the hepatocytes that exceeds 5% of the liver weight, a condition known as steatosis. If untreated, steatosis can lead to steatohepatitis, which can result in fibrosis, cirrhosis and liver failure. Patients with NAFLD complain of fatigue, malaise and feelings of discomfort or “fullness” in the upper right abdomen. Laboratory tests reveal a mild to moderate increase in serum levels of alanine aminotransferase, aspartate aminotransferase or both.[5] The ratio of aspartate aminotransferase to alanine aminotransferase is usually less than 1, but as fibrosis in the liver advances, this ratio increases. NAFLD affects 10 -24% of the world’s population, and 58-74% of obese persons, including 2.6% of children and 23-53% of obese children.[6].
Risk Factors: Insulin resistance and NAFLD are commonly associated with the central obesity phenotype, as visceral fat has a greater lipolytic potential than subcutaneous fat. The fatty acids which are released from visceral fat during lipolysis drain straight into the portal circulation. It is the increased lecels of free fatty acids in the circulation which are thought to be responsible for insulin resistance.[7]
The role of Insulin Resistance in NAFLD: Insulin resistance is thought to be the leading cause of NAFLD. It is thought that fat accumulation in the hepatocytes occurs via lipolysis and hyperinsulinaemia. Microsomal ω-oxidation has been found to produce clinically significant amounts of cytotoxic dicarboxylic acids. This pathway of fatty acid metabolism is closely related to mitochondrial β-oxidation and peroxisomal β-oxidation. A lack in the enzymes associated with peroxisomal β-has been identified as a major cause of steatosis and steatohepatitis. An example of this is deficiency of acyl-coenzyme A oxidase which disrupts the oxidation of long fatty acid chains and dicarboxilic acids, leading to microvesicular steatosis and steatohepatitis. Loss of function of acyl-coenzyme A oxidase also causes sustained hyperactivation of peroxisome proliferator activated receptor-α (PPAR- α), leading to upregulation of PPAR-α regulated genes [8]. Studies suggest that PPAR-α is responsible for promoting the synthesis of uncoupling protein 2 in the liver [9]. Increased levels of fatty acids in the liver provide a source of oxidative stress, which is thought to be responsible for the progression from steatosis to steatohepatitis and finally to cirrhosis.
The role of the keratin cytosleleton Mature and differentiated hepatocytes are the epithelial cells of the liver and normally express type I keratin (keratin 8) and type II keratin (keratin 18), arranged into intermediate filaments in the cytoplasm. In NAFLD, the keratin cytoskeleton in hepatocytes is disrupted, [10] apparently due to oxidative stress.[10]
Endocrine Changes in Obesity
Obesity is associated with changes in the normal endocrine profile, particularly in the sex steroid profile.
Oestrogens: Oestrogens are synthesized by aromatization of circulating testosterones, catalysed by the enzyme aromatase. This occurs at many sites throughout the body, including in adipose tissue [11] [12]. An increase in adipose tissue mass results in a greater capacity for aromatization, and an increase in oestrogen levels [13].
In premenopausal, non-pregnant women, the principal site of aromatization is the ovaries, and only a minor proportion of oestrogen comes from adipose tissue. However, in postmenoapusal women, peripheral aromatisation is enhanced and adipose tissue becomes the main site of oestrogen production [14].
Androgens: The increased capacity for aromatization results in hypoandrogenism in males, as more circulating testosterone is converted to oestrogen [15]. Other factors contributing to the decrease in circulating testosterone include insulin resistance and the suppression of the hypothalamo-pituitary-testicular axis. In contrast, in obese premenopausal women, obesity is associated with an increase in free testosterone levels [16] This is thought to be partly mediated by the increased levels of insulin and IGF-1 associated with obesity.[17] Furthermore, as for oestrogen, adipose tissue is an important site of testosterone production, due to local expression of 17 beta hydroxysteroid dehydrogenase. Therefore there is a positive association between adipose tissue mass and androgen concentration.
SHBG: Sex steroids are highly lipophilic and are carried in the circulation bound to sex hormone binding globulins (SHBG.) Obesity results in a decreased concentration of SHBG [18]. This is thought to be associated with the elevated insulin levels associated with obesity, as insulin inhibits hepatic synthesis of SHBG. The decreased concentration of binding protein results in an increase in the free fraction of sex steroids [16]
| Female Changes | Male Changes | |
|---|---|---|
| Oestrogen | Increase | Increase |
| Androgens | Increase | Decrease |
| SHBG | Decrease | Decrease |
Summary of the endocrine changes in obesity
Obesity and Cancer
It is estimated that 10% of all cancer deaths among non-smokers are related to obesity [18]. It is hypothesised that alterations in hormone metabolism mediate the effects of obesity on cancer risk, due to sex steroid hormone regulation of cell proliferation, differentiation and apoptosis. Many types of cancer are more prevalent in obese subjects, the most widely studied of which are breast and endometrial cancer. These cancers are associated with an increase in oestrogen concentration, a decrease in plasma SHBG and an increase in androgen levels. [12][16] These observations led to the unopposed oestrogen hypothesis, which suggests that mitotic activity of cells is increased in the presence of oestrogen, unopposed by progestogens. [19]. Increased mitotic activity leads to a higher prevalence of mutations, thus increasing the risk of cancer.
Obesity and Infertility
Obesity is thought to account for around 6% of primary infertility [18].
Women: Obese women are at increased risk of anovulatory infertilityCite error: Invalid <ref> tag; invalid names, e.g. too many. This is thought to be due to hyperandrogenism in which high androgen levels increase apoptosis of the granulosa cells, as well as damaging the endometrium and developing oocytes. Excess oestrogen also contributes to infertility by reducing gonadotrophin secretion through excess negative feedback [15]. One of the commonest reproductive disorders in women is PCOS, which affects 5-10% of women of reproductive age [20]. This syndrome is characterized by anovulatory infertility, obesity, hirsutism, multiple ovarian cysts and insulin resistance. Despite their being a well established association between obesity and PCOS, it remains unknown which is the cause and which is the effect.
Men: "Hyperestrogenic hypogonadotropic hypogonadism" in obese men results in high oestrogen levels, low testosterone levels and subfertility. There is a negative association between spermatogenesis and increasing BMI. The mechanism mediating this association is yet to be identified, but it has been suggested that hypoandrogenism in obese males may result in a reduced production of testosterone by the testes. The hyperestrogenism may also cause inappropriate suppression of the hypothalamic-pituitary-gonadal axis, resulting in reduced spermatogenesis. Obesity has also been associated with erectile dysfunction. [15]
Psychosocial Consequences of Obesity
The relationship between obesity and mental health has been the subject of debate over the past 30 years. Early studies were consistent with the “jolly fat” hypothesis, suggesting that obesity confers a protective role against anxiety and depressive disorders, but more recent studies suggest that depression and obesity are linked. [21] [22].
Recent hypotheses linking obesity and aggression: The underlying mechanisms and direction of an obesity-depression link remain largely unknown, although recent research has indicated that gender, obesity severity, comorbid physical illness, stress and abdominal fat distribution are important mediating risk factors for the development of an obesity-mental disorder link.[23] [24] [25] These newly discovered mediators give rise to new hypotheses, involving over-activity of the hypothalamic-pituitary-adrenocortical axis.
Experimental evidence: A nationally representative Canadian study found positive relationships between obesity and an array of lifetime psychiatric disorders and past-year mood and anxiety disorders. These [21] [25] [24][26][27]. Subgroup analyses revealed that obese women were more susceptible to specific mental disorders than men, including depression, mania, panic attacks, panic disorder, social phobia and agoraphobia.[22] These findings, which are consistent with other research, thus reinforce the notion that obese women are more vulnerable to developing mental disorders. This result may be explained in terms of the increased consciousness amongst women to conform to a socially desirable image and weight. The study also positively linked obesity to suicidal behaviours and negatively linked obesity with past-year drug dependence.[28] The former may be attributed to the social stigmatization attached to obesity, inducing feelings of decreased self-worth and decreased self-esteem that fuel suicidal thoughts; and the latter due to protective effects of obesity, arising through food and addictive drugs competing for the same reward sites in the brain [22][29]
Contradictory evidence: Some studies fail to identify mental disorders as a psychosocial consequence of obesity [30][31]. Similarly, whilst much research indicates sex differences between obesity and mental disorders, this is not a universal conclusion. In addition, some research only identifies associations amongst the severely obese, with a BMI of >35kg/m2 [25]. These variable conclusions may reflect methodological differences between studies.
References
- ↑ Boon NA et al. (2006) Davidson's Principles and Practice of Medicine p 111
- ↑ Kopelman PG (2000) Obesity as a medical problem Nature 404:635-42 PMID 10766250
- ↑ Gaal LFV et al.(2006) Mechanisms linking obesity with cardiovascular disease Nature 444:875-9
- ↑ Mathew B et al. {2008) Obesity: effects on cardiovascular disease and its diagnosis J Am Board Fam Med 21:562-8 PMID 18988724
- ↑ Angulo P (2002) Nonalcoholic fatty liver disease New Eng J Med 346:1221-3
- ↑ Angulo P (2007) Obesity and nonalcoholic fatty liver disease, Nutr Rev 65:s57-s63
- ↑ Stranges S et al.(2004) Body fat distribution, relative weight, and liver enzyme levels: a population based study Hepatology 39:754-63
- ↑ Fan Cet al.(1998) Steatohepatitis, spontaneousperoxisome proliferation and liver tumours in mice lacking fatty acyl CoA ocidase; implications for peroxisome proliferator activated receptor alpha ligand metabolism J Biol Chem 273:15639-45
- ↑ Chavin KD et al. (1999) Obesity increases expression of uncoupling protein 2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274:5692-700
- ↑ 10.0 10.1 Zatloukal K (2003) The keratin cytoskeleton in liver diseases J Pathol 204:367-76
- ↑ Purohit A, Reed MJ (2002) Regulation of oestrogen synthesis in postmenopausal women. Steroids 67:979-83
- ↑ 12.0 12.1 Bianchini F et al. (2002) Overweight, obesity, and cancer risk. Lancet Oncol 3:565-74 Cite error: Invalid
<ref>tag; name "Bianchini2002" defined multiple times with different content - ↑ Bates GW, Whitworth N (1982) Effects of obesity on sex steroid metabolism. J Chronic Dis 35:893
- ↑ Simpson ER (2000) Role of aromatase in sex steroid action. J Mol Endocrinol 25:149-56
- ↑ 15.0 15.1 15.2 Hammoud AO et al. Obesity and male reproductive potential J Androl 27:619–25 Cite error: Invalid
<ref>tag; name "Hammoud" defined multiple times with different content - ↑ 16.0 16.1 16.2 Lukanova A et al. (2004) Body mass index, circulating levels of sex steroid hormones, IGF-1 and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 190:161-71
- ↑ Metwally M et al.(2007) The impact of obesity on female reproductive function Obesity Rev' 8:515-23
- ↑ 18.0 18.1 18.2 Haslam DW, James WP (2005) Obesity Lancet 366:1197-209
- ↑ Key TJ, Pike MC (1988) The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk B J Cancer 57:205-12
- ↑ Strauss JF, Dunaif A (1999) Molecular mysteries of polycystic ovary syndrome Mol Endocrinol 13;800-5
- ↑ 21.0 21.1 Stunkard AJ et al. (2003) Depression and obesity Soc Biol Psychiat 54:330-7
- ↑ 22.0 22.1 22.2 Mather AA et al. (2009) Association of obesity with psychiatric disorders and suicidal behaviours in a nationally representative sample. J Psychosomatic Res66:277-85
- ↑ Rivenes, AC et al.(2009) The relationship between abdominal fat, obesity, and common mental disorders: results from the HUNT Study J Psychosomatic Res 66:269-75
- ↑ 24.0 24.1 Simon GE et al. (2008) Association between obesity and depression in middle-aged women Gen Hosp Psychiat 30:32-9
- ↑ 25.0 25.1 25.2 Scott KM et al.(2008) Obesity and mental disorders in the general population: results from the World Mental Health Surveys Int J Obes 32:192-200
- ↑ Carpenter KM et al.(2000) Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study Am J Pub Health 90:251–7
- ↑ Onyike CU et al.(2003) Is obesity associated with major depression? Results from the third National Health and utrition examination survey. Am J Epidemiol 158:1139–47
- ↑ Dong C et al.(2006) Extreme obesity is associated with suicide attempts: results from a family study Int J Obes 30:388-90
- ↑ Kleiner KD et al.(2004) Body mass index and alcohol use J Addict Dis 23:105-18
- ↑ Hasler G et al.(2004) The associations between psychopathology and being overweight: A 20-year prospective study Psychol Med 34:1047–157
- ↑ Faith MS et al. (2002) Obesity-depression associations in the population. J Psychosom Res 53:935–942