Glucose-6-phosphate: Difference between revisions
imported>David E. Volk m (→Fate 1: Pentose Phosphate Pathway: wikilink) |
imported>David E. Volk (structure pictures added, empty WP chemical box removed) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
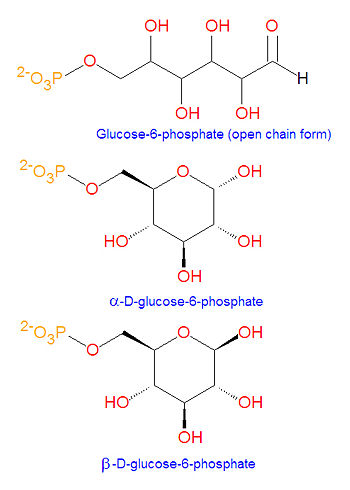
[[Image:Glucose-6-phosphate structures.jpg|right|thumb|350px|{{#ifexist:Template:Glucose-6-phosphate structures.jpg/credit|{{Glucose-6-phosphate structures.jpg/credit}}<br/>|}}Glucose-6-phosphate]] | |||
'''Glucose 6-phosphate''', often abbreviated as '''G6P''', is [[glucose]] sugar [[phosphorylated]] on carbon 6. This compound is very common in [[cell (biology)|cells]] as the vast majority of glucose entering a cell will become phosphorylated in this way. | |||
Because if its prominent position in cellular chemistry, glucose 6-phosphate has many possible fates within the cell. It lies at the start of two major [[metabolic pathway]]s: | Because if its prominent position in cellular chemistry, glucose 6-phosphate has many possible fates within the cell. It lies at the start of two major [[metabolic pathway]]s: | ||
Revision as of 11:44, 7 February 2008
Glucose 6-phosphate, often abbreviated as G6P, is glucose sugar phosphorylated on carbon 6. This compound is very common in cells as the vast majority of glucose entering a cell will become phosphorylated in this way.
Because if its prominent position in cellular chemistry, glucose 6-phosphate has many possible fates within the cell. It lies at the start of two major metabolic pathways:
In addition to these metabolic pathways, glucose 6-phosphate may also be converted to glycogen or starch for storage. This storage is in the liver in the form of glycogen for most multicellular animals, and in intracellular starch or glycogen granules for most other organisms.
Production of glucose 6-phosphate
The major reason for the immediate phosphorylation of glucose is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane, in contrast to free glucose.
Within a cell, glucose 6-phosphate is produced by phosphorylation of glucose on the sixth carbon. This is catalyzed by the enzyme hexokinase in most cells, and glucokinase in certain cells, most notably liver cells. One ATP is consumed in this reaction.
Glucose-6-phosphate is also produced during glycogenolysis from glucose-1-phosphate, the first product of the breakdown of glycogen polymers.
Fate 1: Pentose Phosphate Pathway
When the ratio of NADP+ : NADPH increases, the body realizes it needs to produce more NADPH (a reducing agent for several reactions like fatty acid synthesis). This will cause the G6P to be dehydrogenated by glucose 6-phosphate dehydrogenase. This reversible reaction is the initial step of the pentose phosphate pathway, which generates the useful cofactor NADPH as well as ribulose 5-phosphate, a carbon source for the synthesis of other molecules . Also, if the body needs nucleotide precursors of DNA for growth and synthesis, G6P will also be dehydrogenated and enter the pentose phosphate pathway.
Fate 2: Glycolysis
If the cell needs energy or carbon skeletons for synthesis then glucose 6-phosphate is targeted for glycolysis. Glucose 6-phosphate is first isomerized to fructose-6-phosphate by phosphoglucose isomerase.
This reaction converts glucose 6-phosphate to fructose 6-phosphate in preparation for phosphorylation to Fructose-1,6-bisphosphate. The addition of the 2nd phosphoryl group to produce Fructose-1,6-bisphosphate is an irreversible step, and so is used to irreversibly target the glucose 6-phosphate breakdown to provide energy for ATP production via glycolysis.
Fate 3: Storage as Glycogen
Whenever cellular glucose-6-phosphate levels are high the osmotic pressure inside the cells increases, and this may lead water to diffuse into the cell, causing it to become turgid and (eventually) lyse. Since osmotic pressure is proportional to the concentration of solutes, rather than to the total mass of solutes, this problem can be overcome by storing glucose-6-P in a polymeric form. In plant cells, this polymeric form of glucose is starch, whereas animals store glycogen.
Fate 4: Dephosphorylation and Release into Bloodstream
Liver cells possess glucose-6-phosphatase, which removes the phosphate group from glucose-6-phosphate produced during glycogenolysis or gluconeogenesis. The free glucose is sent into the bloodstream for uptake by other cells.
References
- Berg, Jeremy M.; Tymoczko, Stryer (2002). Biochemistry, 5th. New York: W.H. Freeman and Company. ISBN 0-7167-3051-0.