Delavirdine: Difference between revisions
imported>David E. Volk (New page: {{subpages}} [[Image:Delavirdine structure.jpg|right|thumb|200px|{{#ifexist:Template:Delavirdine structure.jpg/credit|{{Delavirdine structure.jpg/credit}}<br/>|}}Delavirdine, a non-nucleos...) |
imported>David E. Volk m (copyedits) |
||
| Line 2: | Line 2: | ||
[[Image:Delavirdine structure.jpg|right|thumb|200px|{{#ifexist:Template:Delavirdine structure.jpg/credit|{{Delavirdine structure.jpg/credit}}<br/>|}}Delavirdine, a non-nucleoside reverse transcriptase inhibitor for HIV-1.]] | [[Image:Delavirdine structure.jpg|right|thumb|200px|{{#ifexist:Template:Delavirdine structure.jpg/credit|{{Delavirdine structure.jpg/credit}}<br/>|}}Delavirdine, a non-nucleoside reverse transcriptase inhibitor for HIV-1.]] | ||
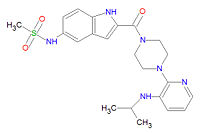
'''Delavirdine''' ('''DLV'''), also called '''SPP''', is an [[antiviral drug]] that is a non-nucleoside, reverse transcriptase inhibitor ([[nNRTI]]) specific for [[HIV]]-1. It binds directly to reverse transcriptase catalytic site and blocks the DNA polymerase activity. HIV-2 RT and eukaryotic (human, for example) DNA polymerases are not effected by this drug. Rashes are the major side effect of delavirdine toxicity, and they should be reported to the attending physician. Rashes typically are resolved in two weeks or less, but severe rash or rash accompanied by fever, blistering, oral lesions, conjunctivitis, swelling, muscle or joint aches should discontinue medication and consult a physician. | '''Delavirdine''' ('''DLV'''), also called '''SPP''', is an [[antiviral drug]] that is a non-nucleoside, reverse transcriptase inhibitor ([[nNRTI]]) specific for [[HIV]]-1. It binds directly to reverse transcriptase catalytic site and blocks the DNA polymerase activity. HIV-2 RT and eukaryotic (human, for example) DNA polymerases are not effected by this drug. Rashes are the major side effect of delavirdine toxicity, and they should be reported to the attending physician. Rashes typically are resolved in two weeks or less, but patients with severe rash or rash accompanied by fever, blistering, oral lesions, conjunctivitis, swelling, muscle or joint aches should discontinue medication and consult a physician. | ||
Its chemical name is N-[2-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazine-1-carbonyl]-1H-indol- | Its chemical name is N-[2-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazine-1-carbonyl]-1H-indol- | ||
| Line 9: | Line 9: | ||
== Drug interactions == | == Drug interactions == | ||
The effect of delavirdine is decreased when used with [[St. Johns Wort]], | The effect of delavirdine is decreased when used with [[St. Johns Wort]], [[antacid]]s, some [[anticonvulsant]]s such as [[carbamazepine]], [[fosphenytoin]], [[methylphenobarbital]], [[phenobarbital]] and [[phentoin]], and with [[rifampin]] or [[rifabutin]]. [[Amprenavir]] and derivatives ([[Fosamprenavir]]) decrease delavirdine levels. | ||
Revision as of 17:08, 31 January 2008
Delavirdine (DLV), also called SPP, is an antiviral drug that is a non-nucleoside, reverse transcriptase inhibitor (nNRTI) specific for HIV-1. It binds directly to reverse transcriptase catalytic site and blocks the DNA polymerase activity. HIV-2 RT and eukaryotic (human, for example) DNA polymerases are not effected by this drug. Rashes are the major side effect of delavirdine toxicity, and they should be reported to the attending physician. Rashes typically are resolved in two weeks or less, but patients with severe rash or rash accompanied by fever, blistering, oral lesions, conjunctivitis, swelling, muscle or joint aches should discontinue medication and consult a physician.
Its chemical name is N-[2-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazine-1-carbonyl]-1H-indol- 5-yl]methanesulfonamide and its chemical formula is C22H28N6O3S (Molecular Mass 456.5611).
Drug interactions
The effect of delavirdine is decreased when used with St. Johns Wort, antacids, some anticonvulsants such as carbamazepine, fosphenytoin, methylphenobarbital, phenobarbital and phentoin, and with rifampin or rifabutin. Amprenavir and derivatives (Fosamprenavir) decrease delavirdine levels.
Delavirdine increases the effects and toxicity of benzodiazepine when taken with alprazolam, midazolam or triazolam and also the effects of some statins, including atorvastatin, lovastatin and simvastatin. The toxicity of ergot may be a concern when delavirdine is taken with the ergot derivatives dihydroergotamine, dihydroergotoxine, ergonovine, ergotamine, methylergonovine, and methysergide. An increased risk of cardiotoxicity and arrhythmias are noted when delavirdine is taken with astemizole, cisapride or terfenadine. The effects of the antiviral drugs indinavir and ritonavir are increased when taken with delavirdine. Increased toxicity is also noted with quinupristin and saquinavir.
External Links
- Delavirdine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).