Coulomb's law
Coulomb's law makes a statement about the forces acting between two electric point charges. The law was first given by Charles-Augustin de Coulomb.
Alternative formulation
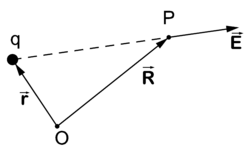
Coulomb's law gives the electric field at the point P due to an electrostatic point charge q at position . The strength of the electric field depends on the inverse-squared distance of P to q and is directed from q to P where the field is measured, that is,
Note that although this may look like an inverse-cubed dependence, we must not forget that the denominator has dimension length. Indeed, defining a (dimensionless) unit vector by
we see more clearly the inverse-squared dependence on distance:
The quantities ε0 and ε0 are the vacuum permittivity and the relative static permittivity (also known as relative dielectric constant), respectively. This formulation of Coulomb's law is in the rationalized SI system of units.
(To be continued)