Coulomb's law: Difference between revisions
imported>Paul Wormer (New page: right|thumb|250px|Coulomb's law. Electric field '''E''' at point ''P'' due to charge ''q''. '''Coulomb's law''' gives the electric field <math>\scriptstyle \v...) |
imported>Paul Wormer (under construction) |
||
| Line 1: | Line 1: | ||
'''Coulomb's law''' makes a statement about the forces acting between two electric point charges. The law was first given by [[Charles-Augustin de Coulomb]]. | |||
==Alternative formulation== | |||
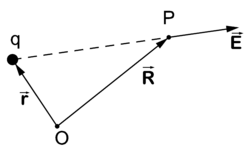
[[Image:Coulombs law.png|right|thumb|250px|Coulomb's law. Electric field '''E''' at point ''P'' due to charge ''q''. ]] | [[Image:Coulombs law.png|right|thumb|250px|Coulomb's law. Electric field '''E''' at point ''P'' due to charge ''q''. ]] | ||
'''Coulomb's law''' gives the electric field <math>\scriptstyle \vec{\mathbf{E}}</math> at the point ''P'' due to an electrostatic point charge ''q'' at position <math>\scriptstyle \vec{\mathbf{r}}</math>. The strength of the electric field <math>\scriptstyle \vec{\mathbf{E}}</math> depends on the inverse-squared distance of ''P'' to ''q'' and is directed from ''q'' to ''P'' where the field is measured, that is, | '''Coulomb's law''' gives the electric field <math>\scriptstyle \vec{\mathbf{E}}</math> at the point ''P'' due to an electrostatic point charge ''q'' at position <math>\scriptstyle \vec{\mathbf{r}}</math>. The strength of the electric field <math>\scriptstyle \vec{\mathbf{E}}</math> depends on the inverse-squared distance of ''P'' to ''q'' and is directed from ''q'' to ''P'' where the field is measured, that is, | ||
:<math> | :<math> | ||
\vec{\mathbf{E}} = \frac{\vec{\mathbf{R}} - \vec{\mathbf{r}}}{4\pi \epsilon_0\epsilon_r|\vec{\mathbf{R}} - \vec{\mathbf{r}}|^3}. | \vec{\mathbf{E}} = \frac{q(\vec{\mathbf{R}} - \vec{\mathbf{r}})}{4\pi \epsilon_0\epsilon_r|\vec{\mathbf{R}} - \vec{\mathbf{r}}|^3}. | ||
</math> | </math> | ||
Note that although this ''may'' look like an inverse-cubed dependence, we must not forget that the denominator has dimension length. Indeed, defining a (dimensionless) unit vector by | Note that although this ''may'' look like an inverse-cubed dependence, we must not forget that the denominator has dimension length. Indeed, defining a (dimensionless) unit vector by | ||
| Line 11: | Line 14: | ||
we see more clearly the inverse-squared dependence on distance: | we see more clearly the inverse-squared dependence on distance: | ||
:<math> | :<math> | ||
\vec{\mathbf{E}} = \frac{\hat{\mathbf{r}}_{qp}}{4\pi \epsilon_0\epsilon_r|\vec{\mathbf{R}} - \vec{\mathbf{r}}|^2}. | \vec{\mathbf{E}} = \frac{q \hat{\mathbf{r}}_{qp}}{4\pi \epsilon_0\epsilon_r|\vec{\mathbf{R}} - \vec{\mathbf{r}}|^2}. | ||
</math> | </math> | ||
The quantities ε<sub>0</sub> and ε<sub>0</sub> are the [[vacuum permittivity]] and the relative static permittivity (also known as relative dielectric constant), respectively. | The quantities ε<sub>0</sub> and ε<sub>0</sub> are the [[vacuum permittivity]] and the relative static permittivity (also known as relative dielectric constant), respectively. | ||
This formulation of Coulomb's law is in the rationalized [[SI]] system of units. | This formulation of Coulomb's law is in the rationalized [[SI]] system of units. | ||
'''(To be continued)''' | '''(To be continued)''' | ||
Revision as of 07:50, 1 February 2008
Coulomb's law makes a statement about the forces acting between two electric point charges. The law was first given by Charles-Augustin de Coulomb.
Alternative formulation
Coulomb's law gives the electric field at the point P due to an electrostatic point charge q at position . The strength of the electric field depends on the inverse-squared distance of P to q and is directed from q to P where the field is measured, that is,
Note that although this may look like an inverse-cubed dependence, we must not forget that the denominator has dimension length. Indeed, defining a (dimensionless) unit vector by
we see more clearly the inverse-squared dependence on distance:
The quantities ε0 and ε0 are the vacuum permittivity and the relative static permittivity (also known as relative dielectric constant), respectively. This formulation of Coulomb's law is in the rationalized SI system of units.
(To be continued)