Chorismate: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (New page: {{subpages}} right|thumb|350px|{{#ifexist:Template:Chorismate DEVolk.jpg/credit|{{Chorismate DEVolk.jpg/credit}}<br/>|}}'''Chorismate''' structure. Choris...) |
imported>David E. Volk mNo edit summary |
||
| Line 4: | Line 4: | ||
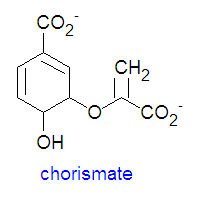
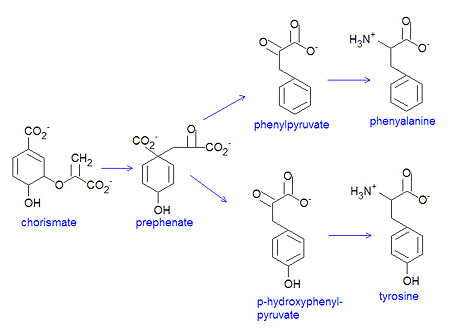
Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids [[phenylalanine]], [[tyrosine]] and [[tryptophan]]. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from [[erythrose-4-phosphate]]. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction. | Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids [[phenylalanine]], [[tyrosine]] and [[tryptophan]]. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from [[erythrose-4-phosphate]]. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction. | ||
[[Image:Chorismate to PHE TYR.jpg|left|thumb| | [[Image:Chorismate to PHE TYR.jpg|left|thumb|450px|{{#ifexist:Template:Chorismate to PHE TYR.jpg/credit|{{Chorismate to PHE TYR.jpg/credit}}<br/>|}}Conversion of chorismate to phenylalanine and tyrosine.]] | ||
Revision as of 14:06, 21 January 2008
Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids phenylalanine, tyrosine and tryptophan. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from erythrose-4-phosphate. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction.