Chorismate: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk mNo edit summary |
imported>Caesar Schinas m (Bot: Update image code) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
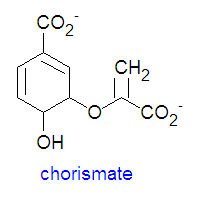
{{Image|Chorismate DEVolk.jpg|right|350px|'''Chorismate''' structure.}} | |||
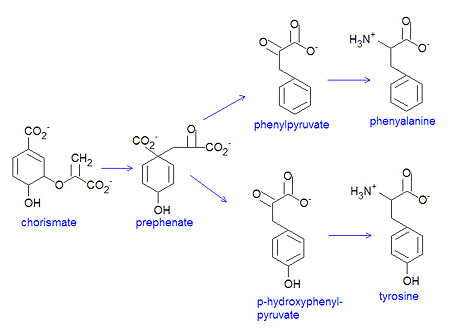
Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids [[phenylalanine]], [[tyrosine]] and [[tryptophan]]. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from [[erythrose-4-phosphate]]. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction. | Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids [[phenylalanine]], [[tyrosine]] and [[tryptophan]]. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from [[erythrose-4-phosphate]]. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction. | ||
{{Image|Chorismate to PHE TYR.jpg|left|450px|Conversion of chorismate to phenylalanine and tyrosine.}} | |||
Latest revision as of 06:33, 8 June 2009
Chorismate is the chemical precursor for the bacterial synthesis of the essential amino acids phenylalanine, tyrosine and tryptophan. The synthesis of phenylalanine and tyrosine occur from the conversion of chorismate to prephenate, at which point their syntheses diverge. Chorismate itself results from a six-step reaction starting from erythrose-4-phosphate. For the synthesis of phenylalanine, chorismate is converted to prephenate by a mutase. Prephenate is then dehydrated and decarboxylated to provide phenylpyruvate. In the synthesis of tyrosine, this step is an oxidative decarboxylation reaction that leaves the hydroxyl group on the aromatic ring. Finally, the α-keto acid is converted to phenylalanine by a transamination reaction.