Chloroform: Difference between revisions
Jump to navigation
Jump to search

imported>Joe Quick m (subpages) |
imported>David E. Volk (add picture, fire hazard) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
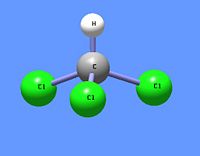
[[Chloroform]] (trichloromethane) is a chlorinated [[methane]] with three chlorine substituents. One of the first [[physician]]s to study and calculate dosages for the use of chloroform as surgical [[anaesthesia]] was [[John Snow (physician)|John Snow]]. | [[Image:Chloroform DEVolk.jpg|right|thumb|200px|{{#ifexist:Template:Chloroform DEVolk.jpg/credit|{{Chloroform DEVolk.jpg/credit}}<br/>|}}Trichloromethane (chloroform).]] | ||
[[Chloroform]] (trichloromethane) is a chlorinated [[methane]] with three chlorine substituents. One of the first [[physician]]s to study and calculate dosages for the use of chloroform as surgical [[anaesthesia]] was [[John Snow (physician)|John Snow]]. However, like [[diethyl ether]], another early anaesthesic, it is very flammable and can lead to explosive fires. This is particularly dangerous in a surgical setting because compressed oxygen is normally present. | |||
Revision as of 16:59, 4 January 2008
Chloroform (trichloromethane) is a chlorinated methane with three chlorine substituents. One of the first physicians to study and calculate dosages for the use of chloroform as surgical anaesthesia was John Snow. However, like diethyl ether, another early anaesthesic, it is very flammable and can lead to explosive fires. This is particularly dangerous in a surgical setting because compressed oxygen is normally present.