User talk:Thomas Wright Sulcer/sandbox2
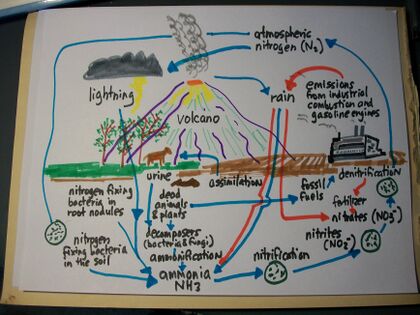
The nitrogen cycle is a complex process involving the transformation and movement of nitrogen into the Earth's atmosphere, by rainfall into the soil, and back again by means of many different chemical processes. It is necessary for life to function since it's a part of amino acids, proteins, and nucleic acids like DNA andRNA, and is a necessary element for photosynthesis in plants.[1] While nitrogen is abundant in air and prevents oxygen from combustion, the air-born nitrogen is generally not useful in its gaseous state to living beings, and it must be transformed to enable its usefulness. Since nitrogen is inert, it requires considerable energy to get the nitrogen out of the air.[2] This occurs naturally as well as by human activity. Nitrogen is an important element in the production of food.
On Earth, atoms of the chemical element, nitrogen (atomic symbol, N; number of protons, 7; standard atomic weight, 14.0067) — an essential element for Earth's living systems — undergo cyclical movement through the atmosphere, the crust (lithosphere), water compartments (hydrosphere), living systems, and non-living organic matter, in a process called the nitrogen cycle, or more specifically, the biogeochemical nitrogen cycle.[3] It's a complex process influenced by natural reactions as well as man-made activity.[4]
When animals defecate or when plants decompose, they produce manure, sewage waste, compost, rotting leaves and sticks and other matter, and the result is waste that contains organic nitrogen. The result is organic soil material called humus. In addition, inorganic nitrogen comes from different sources as well; for example, certain minerals have nitrogen, as well as nitrogen coming from precipitation such as rain or snow, and fertilizers have nitrogen as well. This added nitrogen in the soil makes it easier for living plants to grow healthy. While plants can't use some types of organic nitrogen, it's necessary for microbes living in the soil to convert the organic nitrogen into inorganic nitrogen which enables the plants to use it for their benefit.[4]
Plants use a variety of inorganic or "fixed" nitrogen, or have their nitrogen incorporated into compounds such as nitrate ions, such as:
- Soil-based inorganic nitrogen such as ammonium NH4+
- Urea (NH2)2CO
- Nitrate NO3- (or nitrate ions)[1]
- Nitrite or NO3-[4]Cite error: Closing
</ref>missing for<ref>tag
There is a movement among biogeochemists to see the nitrogen cycle as it relates to the cycles of other compounds in the earth, such as the water cycle, or the cycling of other chemical compounds.[5] A reporter explained:
A biogeochemical cycle is a pathway by which a chemical element, such as carbon, or compound, like water, moves through Earth's biosphere, atmosphere, hydrosphere and lithosphere. In effect, the element is "recycled," although in some cycles the element is accumulated or held for long periods of time. Chemical compounds are passed from one organism to another, and from one part of the biosphere to another, through biogeochemical cycles. Water, for example, can go through three phases (liquid, solid, gas) as it cycles through the Earth system. It evaporates from plants as well as land and ocean surfaces into the atmosphere and, after condensing in clouds, returns to Earth as rain and snow. Researchers are discovering that biogeochemical cycles--whether the water cycle, the nitrogen cycle, the carbon cycle, or others--happen in concert with one another. Biogeochemical cycles are "coupled" to each other and to Earth's physical features.[5]
Notes
View images of nitrogen cycle by right-clicking these links link1 link2 & selecting 'open in new tab'.
See also
References
- ↑ 1.0 1.1 The Nitrogen Cycle, Kimball, 2010-03-26. Retrieved on 2010-03-26. “All life requires nitrogen-compounds, e.g., proteins and nucleic acids.* Air, which is 79% nitrogen gas (N2), is the major reservoir of nitrogen.* But most organisms cannot use nitrogen in this form.* Plants must secure their nitrogen in "fixed" form, i.e., incorporated in compounds such as: nitrate ions (NO3−) ammonia (NH3) urea (NH2)2CO * Animals secure their nitrogen (and all other) compounds from plants (or animals that have fed on plants).”
- ↑ CHM 110 - CHEMISTRY AND ISSUES IN THE ENVIRONMENT, Elmhurst, 2010-03-26. Retrieved on 2010-03-26. “Nitrogen will only react with oxygen in the presence of high temperatures and pressures found near lightning bolts and in combustion reactions in power plants or internal combustion engines. Nitric oxide, NO, and nitrogen dioxide, NO2, are formed under these conditions. Eventually nitrogen dioxide may react with water in rain to form nitric acid, HNO3. The nitrates thus formed may be utilized by plants as a nutrient.”
- ↑ The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air.
- ↑ 4.0 4.1 4.2 The Nitrogen Cycle: Nitrogen Transformations in Soil, Water, and Air, Soil Science Education Home Page (via NASA), 2004. Retrieved on 2010-03-26.
- ↑ 5.0 5.1 Earth's Cycles, Once in Concert, Falling Out of Sync, US News, August 5, 2009. Retrieved on 2010-03-26.