Water
Water is the liquid substance that makes up the bulk of the oceans, lakes and rivers; the liquid that we and other animals, and plants, must take into our bodies to keep them living; and, the liquid we use to wash our bodies and our clothes and put out fires. We learn early in life that we can transform liquid water into solid — ice — by 'freezing' it, and into a gas — steam, water vapor — by boiling it or allowing it to evaporate, and we come to realize that such transformations occur naturally, accounting for glaciers, icy roads, clouds and humid weather.
Water is a chemical compound — a molecule made up of two or more different types of atoms — in the case of water each molecule consisting of the binding together in a particular way the atoms of two of the ninety-two naturally occurring chemical elements, oxygen and hydrogen — two hydrogen atoms binding to one oxygen atom per water molecule. Nevertheless, scientists specializing in particular disciplines (e.g., physics, chemistry, geology, biology) study, describe and/or apply the properties and behaviors of water particular to her discipline.
This article approaches those particulars in a multi-disciplinary, cross-disciplinary and inter-disciplinary way.
The earth sciences perspective
Among the many subjects dealt with by the earth sciences, water presents itself as a major focus and challenge. In its broadest focus it deals with the distribution and masses of water on Earth. Oceanography deals with water in the oceans; hydrology, with water on land and underground; meterology, with the characteristics of water in the Earth's atmosphere; glaciology, with water as glaciers and polar ice; climatology, with the role of water in Earth's climate. Earth scientists also study and teach about the history of water in Earth's history as a planet, and unavoidably, about the possibility of water elsewhere in the Universe.
Water is one of the Earth's basic naturally occurring substances. It covers about 70% of the world's surface…
Addtext....
The chemistry perspective
Chemistry deals with the properties of the water molecule itself, its interactions with other water molecules, and the way those properties and interactions account for its interactions with molecular species other than water — essentially all of its characteristics as 'water'.
The water molecule and its properties
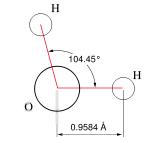
On a molecular level, water is a chemical compound, a 'molecule' comprised of two atoms of hydrogen bonded to one atom of oxygen (H2O) (see figures at left and right). The two types of atoms bond together into a single structure by sharing the unpaired electrons in the outermost electron shells surrounding their nuclei — i.e., by 'covalent' bonds. We can give the least technical explanation of the phenomenon in the following way. Atoms are electrically neutral, having equal numbers of protons (charged positively, situated in the central nucleus) and electrons (charged negatively, surrounding the nucleus in one or more 'shells'). A hydrogen atom has a nucleus with one proton and one (unpaired) electron in its only electron shell. An oxygen has a nucleus with eight protons, with its counterbalancing eight electrons distributed two in its first shell and six in its next and outermost shell — of the latter, two remaining unpaired. For reasons requiring considerations of quantum mechanics, a hydrogen atom behaves as if it 'needed' one more electron to pair up with its sole electron in its first (and only) electron shell, to fill that shell to its natural capacity. An oxygen atom has its first electron shell filled to capacity with two electrons, but behaves as if it 'needed' two additional electrons to pair up with the two unpaired electrons among the six in its next (and outermost) shell, which has a natural capacity of eight electrons. Two hydrogen atoms can thus share electrons with one oxygen atom, filling their respective outermost electron shells to capacity, achieving a more stable state in the covalent bonds.
The accompanying figures model the fact that the two hydrogen atoms do not bond symmetrically about the oxygen atom, but huddle somewhat closer together (~105o) than they would situated oppositely (180o) about the oxygen atom — the explanation of which requires knowledge of 'molecular orbital theory'. The plane of the molecule forms an isosceles triangle. Because the total charge of the nucleus of the larger oxygen atom exceeds that of the two smaller hydrogen atoms, the electrons spend more time nearer the oxygen atom, rendering the charge distribution on the molecule asymmetrical, with more negativity at the oxygen 'pole' of the molecule, and more positivity at the hydrogen 'pole' of the molecule. Chemists refer to the water molecule as 'polar', something roughly akin to the magnetic polar nature of a bar magnet. They also refer to the two covalent bonds in the molecule as 'polar covalent bonds'.
Though quantum mechanically simplistic, the above 'description' of the water molecule can serve to provide a fruitful level of understanding of the properties of water molecules in the aggregate, what we commonly refer to as the liquid, water, as ice, and as the gas, water vapor or steam — discussed in the next section.
Aggregates of water molecules and their properties
When heated to 100 degrees Celsius (its boiling point), water begins to convert to steam, and when cooled to 0 degrees Celsius (the freezing point) it converts to ice. Water is unique among Earth's constituents in that it is the only naturally occurring substance that is found in these three states at temperatures normally occurring on Earth.
When cooled down to 0 K (absolute zero) or as close as can be practically achieved water is the only known substance (as of this date) that has a rest vibrational energy. No other known substance exhibits this peculiar behavior as it was assumed that at the absolute zero temperature every molecule would be totally frozen, having an energy of 0 J/mol.
Additionally, water is usually referred to as "the universal solvent" because of its ability to dissolve more substances than any other existing liquid. It has a neutral acidity, which on a Ph scale has a value of 7.
Frozen water has over 20 crystal structures it can assume dependent upon the circumstances. These water structures are denoted by Roman numbers, I -- XX. The structures of the different ice crystals are reflecting the response of water and its intramolecular interactions to the environment while freezing. This behavior also is specific for water, again making it in this respect an astonishing chemical.
Another special behavior of water is its internal chargedistribution. Water is a polar liquid because of the electronegativity of O2 compared to H2 making the Oxygen atom slightly negatively charged compared to the 2 Hydrogen atoms in the water molecule. Since the electron clouds around Oxygen are pyramidically shaped (compare visual with the diamond structure or the structure of methane) Oxygen can form a pyramidical temporary bond with adjacent water molecules. These quickly forming and dissolving bonds are called hydrogen-bridges because they are energetically substantial yet are formed and broken at a very high rate. These hydrogen-bridges are important in the behavior of water (in all phases). It is the main reason for the high boiling point (373.14 K) and the low freezing point (273.14 K) as well as the exceptional behavior that the density of water is highest at 277.14 K (4˚C) where other liquid chemicals have the highest density at their freezing point.
The exceptional capability of water to dissolve very many chemicals is for a large part due to the structure water can form, breaking and forming hydrogen-bridges all the time and is the major contributor to the biological importance of water.
The anomalies of water
Martin Chaplin has listed the numerous anomalies in the behaviour of water[1]:
Water phase anomalies
1. Water has unusually high melting point.
2. Water has unusually high boiling point.
3. Water has unusually high critical point.
4. Solid water exists in a wider variety of stable (and metastable) crystal and amorphous structures than other materials.
5. The thermal conductivity of ice reduces with increasing pressure.
6. The structure of liquid water changes at high pressure.
7. Supercooled water has two phases and a second critical point at about -91°C.
8. Liquid water is easily supercooled but glassified with difficulty.
9. Liquid water exists at very low temperatures and freezes on heating.
10. Liquid water may be easily superheated.
11. Hot water may freeze faster than cold water; the Mpemba effect.
12. Warm water vibrates longer than cold water.
Water density anomalies
1. The density of ice increases on heating (up to 70 K).
2. Water shrinks on melting.
3. Pressure reduces ice's melting point.
4. Liquid water has a high density that increases on heating (up to 3.984°C).
5. The surface of water is more dense than the bulk.
6. Pressure reduces the temperature of maximum density.
7. There is a minimum in the density of supercooled water.
8. Water has a low coefficient of expansion (thermal expansivity).
9. Water's thermal expansivity reduces increasingly (becoming negative) at low temperatures.
10. Water's thermal expansivity increases with increased pressure.
11. The number of nearest neighbors increases on melting.
12. The number of nearest neighbors increases with temperature.
13. Water has unusually low compressibility.
14. The compressibility drops as temperature increases up to 46.5°C.
15. There is a maximum in the compressibility-temperature relationship.
16. The speed of sound increases with temperature up to 74°C.
17. The speed of sound may show a minimum.
18. 'Fast sound' is found at high frequencies and shows an discontinuity at higher pressure.
19. NMR spin-lattice relaxation time is very small at low temperatures.
20. The refractive index of water has a maximum value at just below 0°C.
21. The change in volume as liquid changes to gas is very large.
Water material anomalies
1. No aqueous solution is ideal.
2. D2O and T2O differ significantly from H2O in their physical properties.
3. Liquid H2O and D2O differ significantly in their phase behavior.
4. Solutes have varying effects on properties such as density and viscosity.
5. The solubilities of non-polar gases in water decrease with temperature to a minimum and then rise.
6. The dielectric constant of water is high.
7. The dielectric constant shows a temperature maximum.
8. Proton and hydroxide ion mobilities are anomalously fast in an electric field.
9. The electrical conductivity of water rises to a maximum at about 230°C.
10. Acidity constants of weak acids show temperature minima.
11. X-ray diffraction shows an unusually detailed structure.
12. Under high pressure water molecules move further away from each other with increasing pressure.
Water thermodynamic anomalies
1. The heat of fusion of water with temperature exhibits a maximum at -17°C.
2. Water has over twice the specific heat capacity of ice or steam.
3. The specific heat capacity (CP and CV) is unusually high.
4. The specific heat capacity CP has a minimum at 36°C.
5. The specific heat capacity (CP) has a maximum at about -45°C.
6. The specific heat capacity (CP) has a minimum with respect to pressure.
7. The heat capacity (CV) has a maximum.
8. High heat of vaporization.
9. High heat of sublimation.
10. High entropy of vaporization.
11. The thermal conductivity of water is high and rises to a maximum at about 130°C.
Water physical anomalies
1. Water has unusually high viscosity.
2. Large viscosity increase as the temperature is lowered.
3. Water's viscosity decreases with pressure below 33°C.
4. Large diffusion decrease as the temperature is lowered.
5. At low temperatures, the self-diffusion of water increases as the density and pressure increase.
6. The thermal diffusivity rises to a maximum at about 0.8 GPa.
7. Water has unusually high surface tension.
8. Some salts give a surface tension-concentration minimum; the Jones-Ray effect.
9. Some salts prevent the coalescence of small bubbles.
The biology perspective
Water by definition
The word "water" itself is practically synonymous with the word "liquid", as we refer to different liquids as water-like: "watered down", or "watery". We know that water moves and flows and is a force; to come across another liquid which visibly resembled water with an unknown chemical makeup, we might infer that it is water but would not know until more evidence was discovered.
Uses
The availability of water on the Earth affords humanity an incredible number of uses, aside from consumption as an integral part of survival. Water can be used to cool machinery and facilities such as nuclear power plants and industrial milling tools. Sometimes isotopes of hydrogen are used for that purpose: Deuterium, which is also known as "heavy water". For obvious reasons not the radioactive Tritium, the third isotope of Hydrogen. Water can also be heated to generate power--the focus of the steam engine which was born out of the industrial revolution, and hydroelectric dams which use water flow and gravity to turn turbines and rotors to generate electricity. Water can also be pressurized, creating a narrow stream that can cut through concrete and steel.
Quality
Water quality is generally determined by measuring the concentration of soluble chemicals, suspended clay particles, and microorganisms. With respect to human health, however, the most important criteria in measuring water quality is the degree of contamination by pathogenic microbes. Because natural water normally contains a large quantity of microorganisms that are nonpathogenic, the sanitary quality of water is determined by the kind, rather than number, of microoganisms.
Furthermore, water quality is measured only by the concentration of indicator bacteria, for measurement of all human pathogens would require too much time, and too many resources. Also, pathogens are difficult to detect due to their small and sporadically changing numbers in water, and their low survival rates (from exposure to water temperature, sunlight, chemicals, and other bacteria). An indicator microorganism is thus one that exists in large numbers in water, has stable properties and a longer survival time, and is absent from uncontaminated water[2].
These criteria are well satisfied when coliforms are used as indicator microorganisms. Coliforms are gram-negative, nonsporeforming, facultative (i.e. non-obligatory) anaerobic, rod-shaped bacteria that ferment lactose with the production of acid and gas within 48 hours at 35°C[2]. Two prominent coliforms are Escherichia coli and Enterobacter aerogenes, both enteric bacteria (that is, of the family Enterobacteriaceae). Along with viruses and protozoan pathogens such as Cryptosporidium, enteric bacteria are one of the major categories of waterborne disease of concern to humans[2]. Enteric bacteria, and in fact many pathogens, are transmitted by a fecal-oral route, and thus the presence of coliforms may indicate pollution by fecal material from humans (or other warmblooded animals).
To this end, coliforms are classified either as total coliforms, or the more specific fecal coliforms, which grow and ferment lactose with the production of acid and gas at 44.5 ± 0.2 °C for 24 ± 2 hours[2]. E. coli, indigenous to the human intestinal tract, is a fecal coliform, while Enterobacter aerogenes can also be found in soil and grain, and is thus considered a nonfecal coliform. Fecal counts are lower, and more clearly indicate fecal contamination by humans and other warmblooded animals than counts of total coliforms in water. E. coli, a major causative agent of the common "traveler's diarrhea," is the most commonly-used indicator microorganism in these counts[2].
Two accepted methods of determining coliform presence in water include the most-probable number (MPN) procedure, and the membrane filter technique. In the former, a series of tests is conducted until a positive result is reached, or a negative result is finally concluded in the last test. The tests involve looking for evidence of gas production under coliform incubation conditions, and observation of the colonies and cells of microorganisms in the water sample. The membrane filter technique uses filters that can select for either total or fecal coliforms, which form colonies on the pads. The more traditional MPN method has the principle advantage of not requiring any prior treatment of the water[2]. Advantages of the membrane filter method include a higher degree of reproducibility, more sensitivity (higher volumes of water can be tested), lower time requirements and fewer steps. The technique is disadvantaged in that the growth of the coliforms can be inhibited by background microorganisms such as cyanobacteria, and adsorbed metals and phenols, on the filter[2].
Treatment
In many parts of the world, water is obtained from a dug well, which draws from a deep pocket under the surface called an aquifer. Areas that use water from shallow aquifers for irrigation may exhibit a distinct rust discoloration due to iron oxidation. However, digging deeper may produce consumable water. The availability of water below the earth's surface and the depth at which it can be reached depends on the water table, which is directly impacted by the geological structure underneath.
In public groundwater and private water supplies in North America, it is desirable that fecal coliforms be virtually absent, while a level of under 10 per 100 ml is desirable in public surface water supplies. Permissible levels for total coliforms in recreational water are under 1,000 per 100 ml, and for fecal coliforms, under 100 per 100 ml[2]. Even very small numbers of pathogens are of concern, since high volumes of water may be consumed by humans. Contamination can result when sewage treatment systems break down, or when post-contamination occurs in pipelines. Against this possibility, residual chlorine is generally left in water after treatment. Such treatment, performed at a water treatment plant, may include the processes of sedimentation (flocculation), filtration, and disinfection[2] with arsenic, fluoride, and other additives. (In the case of fluoride, the treatment may help prevent tooth decay.) Pathogens such as Giardiasis and Cryptosporidium, however, produce cysts that are resistant to chlorination, and can only be removed by sedimentation and very fine filtration — through slow sand filters, for example[2].
In recent decades a market for "bottled water" has developed. This water is often advertised to have been additionally filtered, or to have come from a spring in order to enhance its "purity". The benefit of this bottled water has been disputed for a long time. Many governments are questioning the pollution created by the plastic bottles and are considering a ban on these plastic throw away bottles. Other types of water sold may have additives such as flavors, caffeine, or natural herbal supplements.