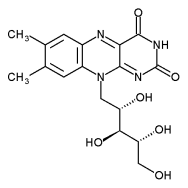
Flavin
- Flavin is also the name of a commune in the Aveyron département, in France. Flavin is also a minor character of Andromeda TV series.
Flavin is a tricyclic heteronuclear organic ring based on pteridine whose biochemical source is the vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and in other circumstances, is found as flavin mononucleotide (or FMN), a phosphorylated form of riboflavin. The flavin group is capable of undergoing oxidation-reduction reactions, and can accept either one electron in a two step process or can accept two electrons at once. In the form of FADH2, it is one of the cofactors that can transfer electrons to the electron transfer chain. Since FMN fluoresces more than FAD, these flavins can be readily distinguished experimentally through a simple fluorescence-based assay. In this assay, the 530 nm fluorescence (upon a 360 nm excitation) of a sample solution is measured before and after treatment with Naja naja venom. Under these conditions the fluorescence of FMN solutions does not change, but the phosphodiesterases present in the venom cleave FAD into FMN and adenine, yielding a 10-fold increase of fluorescence of FAD solutions [1].
FAD
Flavin adenine dinucleotide is a cofactor in the enzymes monoamine oxidase, D-amino acid oxidase, glucose oxidase, and xanthine oxidase.
FADH / FADH2
FADH and FADH2 are (respectively) one-electron and two-electron reduced forms of FAD. FADH2 is produced in the citric acid cycle. In oxidative phosphorylation, one molecule of FADH2 yields approximately 1.5 ATP
FMN
Flavin mononucleotide is a prosthetic group found in NADH dehydrogenase. File:Flavin.png
See also
References
- Voet, D.; Voet, J.G. (2004). Biochemistry (3rd ed.). John Wiley & Sons. ISBN 0-471-39223-5