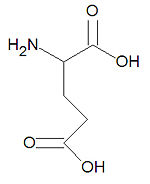
Glutamic acid
Glutamic acid, also called glutamate and abbreviated as Glu or E, is one of the twenty common amino acids used by living organisms to build proteins. It is one of only two acidic amino acids, the other being aspartic acid, and it is similar to glutamine which has an amide function in place of the acid. Being hydrophilic, glutamate is often found on the surfaces of proteins. Glutamate also serves as an energy source in the brain, is the chemical precursor for the amino acids glutamine, proline and arginine, and most of the amino acids derive their Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha} -amino nitrogen atom from the glutamate Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha} -amino nitrogen by transamination.
synthesis and conversion of glutamic acid
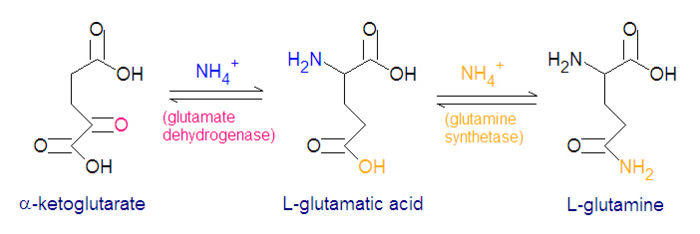
The enzyme glutamate dehydrogenase catalyzes the reaction between an ammonium ion and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha} -ketoglutarate to form L-glutamic acid. This reaction is reversible.
- NH4+ + Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha} -ketoglutarate + NADPH + H+ → L-glutamate + NADP+ + H20
Three amino acids are derived from glutamate, namely glutamine, proline and arginine. The enzyme glutamine synthetase catalyzes the reaction between glutamate and ammonium ion, with energy derived from the hydrolysis of ATP. Nitrogen metabolism is largely controlled by glutamine synthetase and glutamate dehydrogenase which are present in all living organisms, and most procaryotes also have the enzyme glutamate synthase to catalyze the reductive amination of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha}
-ketoglutarate.
- glutamate + NH4+ + ATP → glutamine + ADP + Pi + H+
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha} -ketoglutarate + glutamine + NADPH + H+ → 2 glutamate + NADP+
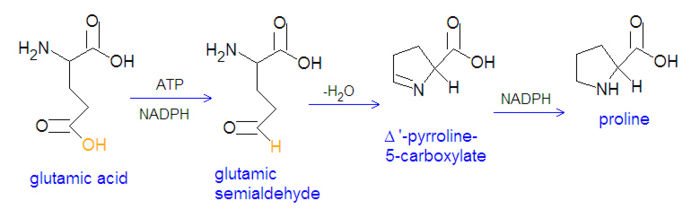
The non-essential amino acids proline and arginine are derived from glutamate through several steps. First, glutamate is converted to an acyl phosphate by reacting with ATP, and the acyl phosphate is reduced to glutamatic-γ-semialdehyde by NADPH. The semialdehyde can be converted to ornithine, which is a precursor of arginine, or it can cyclize to from Δ'-Pyrolline-5-carboxylate, which can be reduced by NADPH to from proline.