Bariatric surgery
Currently bariatric surgery is the most consistently effective treatment for obesity. This article discusses the specific bariatric operations and report how surgery affects hormonal modulation of both the gut-brain and adipose-brain axes.
For many severely obese patients, bariatric surgery has proved to be the only effective method in the long term treatment of obesity. Bariatric surgery works by altering the anatomy of the gastrointestinal tract. This limits the volume of food the stomach can hold, interferes with the absorption of calories, and interferes with orexigenic and anorexigenic signalling between the gut, adipose tissues and the brain. Bariatric surgical procedures are continually evolving, and some of the procedures currently in use are described here.
Bariatric Surgical Procedures
Restrictive Surgery
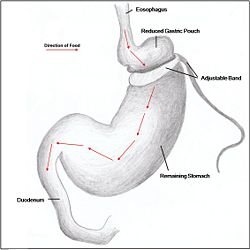
Restrictive surgery reduces the size of the stomach causing decreased hunger, and increased satiety following ingestion of food. Surgical procedures include gastric banding (Fig. 1), where an adjustable band is clipped just below the cardia of the stomach creating a small gastric pouch above (kerrigan, le roux). Other forms of restrictive surgery include gastroplasty, also called 'stomach stapling', and sleeve gastrectomy, where a large part of the stomach is removed.
Malabsorptive Surgery
Jejuno-ileal bypass, duodenojejunal bypass and biliopancreatic bypass have, in the past, all been performed to reduce the amount of calories absorbed by reducing the size of the stomach. However, as a result of extreme malnourishment these procedures are now rarely performed. Instead, duodenal switches are the dominant form of malabsorptive surgery. Moreover, the procedure is usually accompanied with a restrictive form of surgery to have a greater effect on weight loss. Such forms of combination surgeries are most commonly performed.
Combination Surgery
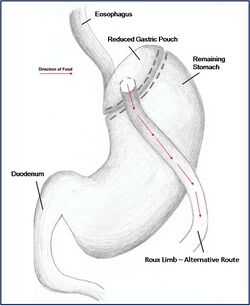
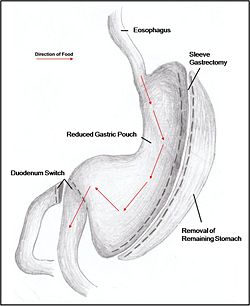
Combination surgery combines the benefits of both restrictive and malabsorptive surgery. The most common bariatric procedure in the USA is the Roux-en-Y Gastric Bypass (RYGP) (Fig. 2). Most restrictive procedures are performed in conjunction with a duodenum switch. For instance, a sleeve gastrectomy reduces the stomach size drastically while the additional switch of the duodenum reduces the amount of calories absorbed (Fig. 3).
The Efficacy of Surgery
There is substantial weight loss following bariatric surgery. A meta-analysis of studies of 10,172 patients [1] reported that, on average, patients lose 61% of their weight; bilopancreatic diversion and duodenal switch procedures produce most weight loss (70% weight loss), followed closely by gastroplasty (68.2%) and gastric bypass (61.6%), with gastric banding ranked lowest (48%). Bariatric surgery also improves several obesity related comorbidities which may prove to be of greater benefit than weight loss to the individual. An improvement or resolution of type 2 diabetes or impaired glucose tolerance was found following all types of bariatric surgeries. A resolution of diabetes allows patients to end their diabetes associated medication and improved glucose tolerance allows patients to maintain normal levels of blood glucose. For patients with diabetes before surgery, 76.8% resolved and 86% improved their diabetes with biliopancreatic diversion and duodenal switch procedures ranking highest (99% resolution), followed by gastric bypass (84%), gastroplasty (72%), and lastly gastric banding (48%). Hyperlipidemia (high levels of lipids or lipoproteins in the blood), hypercholesterolemia (high blood cholesterol) and hypertriglyceridemia (high levels of triglycerides in blood) also improve following all bariatric surgeries, with reported improvements of 70% or better. Greatest improvements are seen following biliopancreatic diversion, duodenal switch and gastric bypass procedures.
Hypertension (high blood pressure) also improves following all bariatric surgeries. A meta-analysis reported that 79% of patients show improvement in their hypertension and, of 4,805 patients studied, 62% resolved their condition. The efficacy rankings of each surgery procedure vary in terms of improvement or resolution.
Obstructive sleep apnea (a sleep disorder characterised by pauses in breathing caused by obstructed airways) and obesity hypoventilation syndrome improve following all bariatric procedures, with 86% of patients resolving their sleep apnea.
Surgical Effects Regarding Gut Hormones
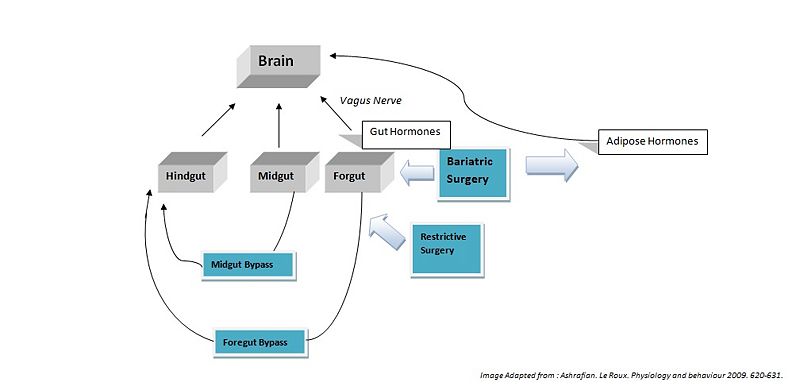
Hindgut Hormones[2]
Peptide tyrosine tyrosine (PYY) is a peptide belonging to the neuropeptide Y (NPY) family [2]. PYY is normally released in proportion to calorie intake and has recently been described as an important feedback molecule in the Gut-Brain axis. As well as being a satiety signal, PPY has also been suggested to increase energy expenditure [3]. Following bypass and restrictive surgery, basal and postprandial PYY levels increase [4]. In addition, basal PYY levels increase after ileal resection, and patients have been reported to have lost weight [5]. By contrast, diet-induced weight loss did not increase PYY levels [6].
Glucagon-like peptide-1 (GLP-1), like PYY, as a circulating peptide hormone that acts on the hypothalamus as an anorexigenic signal. Most patients show a marked increase in basal and postprandial concentrations of GLP-1 after foregut bypass surgery. Midgut bypass also increases GLP-1 secretion, but studies on restrictive surgery have shown either no change or a decrease in basal levels of GLP-1. Of specific interest GLP-1 has been shown to improve postprandial glycaemic control, being a potent incretin mimetic. A GLP-1 agonist, exenatide, has not yet been approved for use as an anti-obesity drug, but is being used to treat type 2 diabetes mellitus[7].
Midgut Hormones[3]
Neurotensin (NT), is an anorexigenic signal whose expression is downregulated in ob/ob (leptin deficient) mice. Following bypass surgeries (5 of 6 reported) an increase in NT levels has been demonstrated which could contribute to the anorexigenic effects [8].
Foregut Hormones[4]
Ghrelin is an orexigenic hormone released from the fundus of the stomach. As a result, ghrelin levels increase when calorie intake is reduced during dieting. However, of 42 studies following foregut bypass surgeries, only 50% reported a decrease in ghrelin release. Many of these studies reported ghrelin levels immediately after surgery, whereas studies of long term ghrelin change following surgery show no significant trend. Results for restrictive surgeries show only an 18% decrease in ghrelin levels. These results indicated that bariatric surgeries may not directly modulate ghrelin release [9] [10].
Cholecystokinin (CKK) was the first anorexigenic gut peptide to be implicated in the control of appetite [11]. One would speculate that CCK would rise following bariatric surgery however studies suggest no role in the mediation of CCK following bariatric surgery [12].
Glucose-dependent Insulinotrophic Peptide (GIP) is a hormone produced in the foregut, and released when simple, energy-dense nutrients like glucose and free fatty acids are present and it promotes nutrient (energy) storage [13]. Those with obesity and diabetes often have a diet-induced, chronic elevation in GIP levels with peripheral tissue receptor down-regulation that is analogous to insulin insensitivity. Therefore, it would be optimal to decrease GIP levels and improve GIP sensitivity to lose weight. Diet-induced weight loss increases GIP levels after a nutrient-rich meal and prevents further weight loss because the body counters the perceived starvation by increasing nutrient deposition in central and peripheral stores. By contrast, proximal foregut bypass surgery, roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch decrease GIP levels.
Surgical Treatment as Opposed to Diet
It is still not clear exactly how bariatric surgery works, but is thought to increase energy metabolism, reduce orexigenic signals, increase anorexigenic signals and to interfere with calorie absorption. For instance, after diet-induced weight loss orexigenic hormones like ghrelin are released in abundance as the body is in 'starvation mode' - increasing the chances of the individual to over-eat to regain energy therefore regaining the weight. However, even though bariatric surgery similarly induces weight loss - the reduction in stomach size allows a proportionate decrease in ghrelin released. Hence, although there is reduced calorie intake, less orexigenic signals are released. [14]
Under fasting conditions, most obese people have lower PYY levels than those who are lean. A year after bariatric surgery, such as jejunoileal bypass and vertical banding, the levels of fasting PYY are increased. Moreover, concentrations within an hour of eating did not seem to change considerably - whether in an obese, lean, or postoperative. However, after Roux-en-Y gastric bypass, there is a postprandial PYY surge. There are no clear indications as to what might lead to this, but it is believed that the reason for this exaggerated release is the removal of the pylorus (as well as a portion of the stomach) that allows food to enter the small intestine more quickly. This early rise in PYY levels will therefore also induce satiety early. Again, although such cases have been reported, controversy still surrounds the exact mechanisms and effects of surgery on PYY levels due to the variability between individuals. There have not been enough postprandial PYY results from larger number of individuals that with strong statistical indications can correlate significant changes to satiety induction as a result of PYY level alterations post-operatively[15].
Type 2 diabetes develops in about 41% of obese patients as the body becomes resistant to insulin - the correlative link between obesity and diabetes is now more commonly termed as Diabesity. Diet induced weight loss ameliorates the insulin response but not to the extent where euglycemia is restored. Furthermore, the usual cases where weight is re-gained after diets will almost certainly lead to the re-establishment of insulin resistance. After biliopancreatic diversion with Roux-en-Y gastric bypass, insulin sensitivity improves greatly. Within a year after bariatric surgery, however massive the weight loss achieved, patients are usually still obese (with a BMI of ~30 kg/m2), but improvements of insulin resistance occur a few months after the operation. Therefore, the initial improvement of type 2 diabetes following bariatric surgery seems to take place while the patient is still obese.[16].
References
- ↑ Buchwald H et al. (2004) Bariatric surgery: A systematic review and meta-analysis JAMA 292:1724-37
- ↑ (Broberger,2005)
- ↑ (Murphy)
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiology and Behaviour 97:620-31 [1]
- ↑ Broberger C (2005) Brain Regulation of food intake and appetite: molecules and networks J Int Med 258:301-27
- ↑ Olivan B et al. (2009) Effect of weight loss by diet or gastric bypass Surgery on peptide YY3-36 levels. Annals of Surgery 249:948-53
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ Korner J et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90:359-65
- ↑ Holdstock C et al. (2003) Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88:3177-83
- ↑ (murphy)
- ↑ Ashrafian H, le Roux CW (2009) Metabolic surgery and gut hormones - A review of bariatric entero-humoral modulation Physiol Behav 97:620-31
- ↑ (Rosen, 2009)
- ↑ Korner J et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin". J Clin Endocrinol Metab 90:359-65
- ↑ Korner (2005)
- ↑ Polyzogopoulou EV et al. (2003) Restoration of euglycaemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery Diabetes 52:1098-103
DeMaria (2007) Bariatric surgery for morbid obesity. N Eng J Med 356:2176-83 Murphy KG Gut Hormones in the control of appetite Exp Physiol 89:507-616