Fenske equation
The Fenske equation is used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a distillation column that is being operated at total reflux (i.e., which means that no overhead product is being withdrawn from the column). The derivation of the Fenske equation assumes that the relative volatility is constant in the distillation column.
Theoretical plates are also often referred to as theoretical trays or equilibrium stages.
The equation was derived by Merrell Fenske in 1932 [1], a professor who served as the head of the chemical engineering department at the Pennsylvania State University from 1959 to 1969.
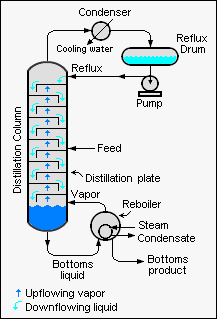
When designing large-scale, continuous industrial distillation towers, it is very useful to first calculate the minimum number of theoretical plates required to obtain the desired overhead product composition.
A common version of the Fenske equation
This is one of the many different but equivalent versions of the Fenske equation:[2][3][4][5]
where:
| = minimum number of theoretical plates required at total reflux (of which the reboiler is one) | |
| = mole fraction of more volatile component in the overhead distillate | |
| = mole fraction of more volatile component in the bottoms product | |
| = average relative volatility of more volatile component to less volatile component |
For ease of expression, the more volatile and the less volatile components are commonly referred to as the light key (LK) and the heavy key (HK), respectively. Using that terminology, the above equation may be expressed as:[3]
or also:
If the relative volatility of the light key to the heavy key is constant from the column top to the column bottom, then is simply . If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:[2]
| where: | |
| = relative volatility of light key to heavy key at the top of the column | |
| = relative volatility of light key to heavy key at the bottom of the column |
The above form of the Fenske equation can be modified for use in the total reflux distillation of multi-component feeds.
Another form of the Fenske equation
Using Raoult's law and Dalton's law for a series of condensation and evaporation cycles (i.e., equilibrium stages or theoretical plates), another form of the Fenske equation is obtained for use in gas chromatography:[6]
| where: | |
| = number of equilibrium stages | |
| = mole fraction of component n in the vapor phase | |
| = mole fraction of component n in the liquid phase | |
| = vapor pressure of pure component n |
and and denote the more volatile and the less volatile components, respectively.
Shortcut calculations for designing distillation columns
There are many so-called shortcut calculation methods for designing industrial distillation columns. The most commonly used one is the Fenske-Underwood-Gilliland method.
The Fenske equation estimates the minimum number of theoretical plates or equilibrium stages at total reflux. The Underwood equation[7] estimates the minimum reflux for an infinite number of theoretical equilibrium stages. The Gilliland method[8] then uses Fenske's minimum plates and Underwood's minimum reflux to estimate the theoretical plates for a given distillation at a chosen reflux.
The estimates such as provided by the Fenske-Underwood-Gilliland shortcut calculations are most effective when used to obtain a preliminary design before following up with the use of distillation simulation software which utilize much more rigorous calculation methods.
References
- ↑ M.R. Fenske (May 24, 1932). "Fractionation of Straight-run Pennsylvania Gasoline". Industrial Engineering Chemistry 24 (5): 482-485.
- ↑ 2.0 2.1 Distillation notes (Loren Schreiber, Florida State University)
- ↑ 3.0 3.1 David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9. Google books Search for "Fenske" to get to page 200.
- ↑ Tutorial 6: Separation Processes (J. Skilling, University of Edinburgh, Scotland)
- ↑ Maxwell, J.B. (1950). Data Book on Hydrocarbons, 1st Edition. D. Van Nostrand.
- ↑ Separation By Distillation With Quantitative Gas Chromatography (08-18-09), received as a personal communication from the Chemistry Department of the U.S. Naval Academy, which is not available online.
- ↑ A.J.V. Underwood (1948). Chemical Engineering Progress, Vol. 4:603.
- ↑ E.R. Gilliland (1940). Industrial Engineering Chemistry, Vol.32:1220.
![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {X_{d}}{1-X_{d}}}{\Big )}{\Big (}{\frac {1-X_{b}}{X_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/06af3d7fcd0e4cac0751fb951d065cca1c4fcaf5)




![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {LK_{d}}{HK_{d}}}{\Big )}{\Big (}{\frac {HK_{b}}{LK_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/98b652ba920414b056ca83cd3a8b1de0f0bb1c90)
![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {LK_{d}}{1-LK_{d}}}{\Big )}{\Big (}{\frac {1-LK_{b}}{LK_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0301b1202e7c9a2987bffe2f768ddf3fcdc324c0)










