Oxidative stress
Chemists and biologists typically define oxidative stress as an imbalance, in biological cells in particular, between the state of two chemical processes—
- the formation of 'reactive oxygen species' (ROS), more generally 'reactive species' (RS), [1] some of which qualify as 'oxygen free radicals' — potent 'oxidizing' (viz,. electron-capturing) atoms, ions or molecules; and,
- the elimination or 'reduction' of those oxidizing agents by 'antioxidants' — 'reducing' (viz., electron-donating) atoms, ions or molecules;
— the imbalance characterized by an excess of the former or a deficiency of the latter, or both, leading to an alteration of a cell's 'redox' state towards the 'oxidized' state.
A leading researcher defines oxidative stress:
The term ‘oxidative stress’ refers to a serious imbalance between RS [reactive chemical species] production and antioxidant defences.[2]
Oxidative stress, therefore, may result from diminished antioxidant defense, excessive production of reactive oxygen species, or a combination of both.
The concept of oxidative stress occupies central importance in biology, as it applies, depending on circumstances, either to physiological phenomena essential for optimal functioning of organisms or to pathophysiological phenomenona, such as cardiovascular diseases, cancer and other clinical disease states, as well as to considerations of the mechanisms underlying aging. [3]
Some key points about oxidative stress:
- Currently, the core of the definition of oxidative stress: An imbalance between 'oxidant' and 'antioxidant' activity, favoring the 'oxidants', potentially causing cellular injury.
- Experimental evidence suggests that, for a living system adapted to uptake and utilization of oxygen, the beneficial effects separate themselves narrowly from the potentially injurious effects produced by oxygen-linked 'oxidation reactions'.
- Thus, normal physiological processes that involve oxidation reactions, such as anti-microbial defense, acute inflammation and normal signaling processes, share underlying mechanisms with pathophysiological phenomena, such as chronic inflammation, aging, carcinogenesis, atherosclerosis, and drug toxicity. It seems that for organisms living an aerobic ("living only in the presence of oxygen"[4]) existence entails passage between Scylla and Charybdis.
- 'Oxidative challenge' does not necessarily lead to oxidative damage.
- When it does, treatment involves efforts to increase the antioxidant side of the balance, by limiting the culprit oxidative reactions, by countering the culprits with increased antioxidant defenses, or both.
In the definition of oxidative stress given above, and in the key points list, the terms embraced by single quotes will require definition and further discussion before any real understanding of the concept of oxidative stress can emerge. Accordingly, we will try to explicate those sub-concepts in turn, and relate them in a synthesis of the concept of oxidative stress, its causes, beneficial functions, and its pathological consequences.
Free radicals
In this section we begin the explication of oxidative stress by elaborating on the following assertions about 'free radicals', a major cause of biological oxidative stress:
- A free radical is any chemical species (e.g., atoms, ions, molecules), capable of independent existence — hence the term ‘free’ — that contains one or more unpaired electrons typically in its outermost or 'valence' electron shell.[5]
- An unpaired electron is one that occupies an atomic or molecular orbital without an accompanying electron — orbitals have a maximal capacity for holding two electrons.
- A superscript dot is usually used to denote the unpaired electron.
- Radicals can form in many ways, in both industrial and biological systems, and in the latter, may form because of substances in food, water, pesticides, pollutants including tobacco smoke, drugs and radiation; they may form in biological cells because of normal responses to microbial infection, and as a byproduct of mitochondrial function.
- Because of their unpaired 'valence' electrons, radicals possess a high degree of chemical reactivity and potential for disrupting the structure and activity of biologically important molecules.
- A full understanding of free radical chemistry requires understanding many concepts from quantum mechanics and quantum chemistry, among which include:
- Atomic orbitals
- The Pauli principle
- Spin quantum numbering
- Covalent bonding
- Molecular orbitals
- Hund's rules
Chemists define a 'free radical' as an atom, ion or molecule that has one or more unpaired electrons, whereas all its other electrons, if it has others, exist in pairs that have 'opposite spin', technically opposite 'intrinsic angular momentum' or differing spin quantum numbers (+½ and -½).[6] The qualifier 'free' denotes the independent existence of the radical species, and many biological chemists drop the term as they consider only those radicals which can exist independently, however briefly.
ABCs of atomic structure
A little background on the structure of atoms will help explain the special properties of radicals. After British scientist Joseph John (J. J.) Thomson (1856-1940) [7] [8] discovered the negatively charged electron, the British physicist Ernest Rutherford (1871-1937) discovered the positively charged nucleus and conceived of an atom as low-mass negatively charged electrons in orbits around a more massive nucleus in the center, like planets orbiting a sun. Between 1913 and 1915 the Danish physicist Niels Bohr (1885-1962) developed a model of the atom that proposed that electron orbits could occupy only certain positions around the nucleus. Those positions came to be conceived as 'shells' successively more distant from the nucleus and having successively greater energy. Then in 1923 the French physicist Louis de Broglie (1892-1987), thinking about how one could view light as either particles (photons) or waves (electromagnetic waves), proposed that electrons might have wave-like properties. The Austrian physicist Erwin Schrödinger (1887-1961) showed in 1926 that one could treat an electron as a wave occupying a so-called 'orbital', strictly a mathematical 'wavefunction' that gave the probability of the electron's location everywhere in the space around the nucleus. The modern description of the atom takes as fundamental that electrons occupy orbitals within their shells that have distinctive shapes indicating likely volumes of electron occupancy — mathematicians refer to them as spherical harmonic functions — the surfaces of the shapes enclose the region of space that likely contains the electron. Physicists and chemists designate the differently shaped orbitals with the lowercase letters s, p, d, f, and others, and the successive shells by the uppercase letters K, L, M, N, or the successive numerals 1,2,3,4....
We can imagine an atom's electron orbitals as imaginary enclosures of particular shapes depicting where electrons likely spend most of their life 'surrounding' their nucleus. The 'shapes' of the enclosures reflect three-dimensional mathematical wavefunctions. The wavefunctions have algebraic sign (positive, negative), analogous to the peaks and troughs of a water wave, often represented in visual depictions as colors (e.g., red, blue). The two different 'colors' of electron distribution in an orbital have significance in understanding molecular orbitals, the types of orbitals electrons occupy in molecules. "The recognition by quantum mechanics that an electron distribution has "color" is one of the reasons for the success of that theory in explaining chemical reactions: classical physics was "colorblind," and unaware that the distribution of an electron has a "color" as well as a shape." [9]
In 1924, the Austrian-American physicist Wolfgang Pauli (1900-1958) inferred that only two electrons can occupy an orbital, formulating his 'exclusion principle'. In 1925, the Dutch-American physicists, Uhlenbeck and Goudsmit, discovered that the two electrons must differ in a property interpreted in classical physics terms as spin, as if each electron rotated on a spherical axis, in one of two directions, one clockwise (↑), the other counterclockwise (↓). Soon after Pauli explained the quantum mechanical foundation of this discovery.
Given the two-electrons-only capacity of orbitals — Pauli's exclusion principle — the electrons of atoms with many electrons (e.g., carbon has 6, oxygen 8), arrange themselves in concentric shells about the nucleus with differing numbers of two-electron-capacity orbitals. The first shell, the innermost shell, has only one orbital, an orbital with the shape designated s (spherical), and the orbital itself designated a 1s orbital, in honor of its principal quantum number n = 1, giving it the 'first' shell label and the lowest energy level designation. The second shell has both s- and p-type orbitals, 1 s-type, 3 p-types labeled px, py, and pz. Thus an atom with electrons fully occupying the first and second shells would have two 1s electrons (1s2), two 2s electrons (2s2), and six 2p electrons (2p6), for a total of ten. With 10 balancing protons in the nucleus (atomic number, Z=10), that characterizes neon, an atom with a 'closed', or 'complete', set of shells, and therefore quite unreactive chemically.
The following table summarizes certain main features of atomic structure in relation to orbitals:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
In the table, note that the different orbital types in each shell segregate as 'subshells'.
Consider the most common variety (also known as isotope) of hydrogen atom, which comprises a nucleus consisting of one proton, a positively charged particle, and one electron, a negatively charged particle, the latter occupying a surrounding electron shell, having no sub-shells, a shell that has the intrinsic capacity to hold two electrons. We can symbolize it as H•, or H•. For quantum mechanical reasons relating to a kind of stability, so to speak, the hydrogen atom tends to behaves as if it wanted two electrons in that shell, specifically a pair of electrons 'spinning' in opposite directions. Accordingly, the hydrogen atom tends to react with another hydrogen atom like itself, one with an oppositely spinning electron in its only electron shell, such that the two hydrogen nuclei share the two electrons in the shell, binding the two atoms together in what chemists refer to as a 'molecule' with a 'covalent bond' — in this case, a non-polar covalent bond, as no electrical asymmetry persists in the molecule because the equal positive charges on the two hydrogen nuclei means the orbiting electrons spend equal time near each nucleus. We can symbolize the hydrogen molecule as H:H, depicting the shared electrons between the two nuclei, or more simply, as H–H or H2. A hydrogen atom may satisfy its reluctance to possess an unpaired electron also by simply donating its electron to some other electron-eager atom or molecule, and continue its existence as a hydrogen ion, H+. In water, H2O, the medium of biological chemistry, some H+ can dissociate from the water molecule, leaving a hydroxyl ion, OH− in its wake.
Referring then to our definition of 'free radical', or just 'radical', we can recognize the hydrogen atom, H•, as a radical, and we can begin to appreciate the reactive nature of a radical eager to achieve electron pairing. We will see that other radicals, like H•, may through transfer or sharing, either lose an electron, or gain one, depending on circumstances.
Some definitions
- At this point we introduce a few helpful definitions:
|
|||||||||||||||||||||||||||||||||
Molecular structure
Consider next an atom with at least two more nuclei and electrons than the hydrogen atom. The additional electrons must locate in shells 2 and above (see Table: Features of Atomic Structure in Relation to Orbitals). The higher the shell the higher the energy levels of the electrons there, farther away from the positively charged nucleus. Each higher numbered shell successively has a greater number of orbitals of a greater number of orbital types.
To quote chemist Peter Atkins, "The central quantum mechanical idea on which the modern description of the structure of the atom is based is that its electrons occupy 'orbitals." The electrons occupying the outermost shell, whether the shell has only 1 electron or as many as it can carry, act as so-called 'valence' electrons, the electrons largely responsible for the determining whether the atom can form a bond with another atom, and the strength of the bond. In biological systems, the action in chemical reactions takes place with the valence electrons, in the outermost shell.
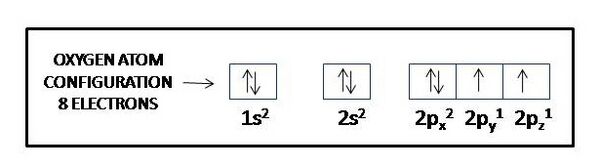
Consider specifically the oxygen atom, which plays a central role in oxidative stress. It has 8 protons and 8 electrons. An electron configuration table for the oxygen atoms reads as follows:
- 1s2
- 2s2 2p4
We interpret the table entry as indicating 2 electrons in the first shell, and 6 in the second. In the second shell, two electrons occupy an s-type orbital, and 4 occupy p-type orbitals. Given that a p-type orbital has a capacity of 6 electrons (viz., a 2 electron capacity each in px, py, and pz), oxygen falls short by two electrons of its 'wanting' to fill its outermost, or valence, shell, shell #2, to its full natural capacity. In biological systems an oxygen atom typically finds itself sharing two electrons originally fully owned by one or two other atoms, sharing them in covalent bonds. For example, it can share the eager-to-pair-up single 1s electrons in two hydrogen atoms, forming the covalent bonds that make up a water molecule, H:O:H, or H-O-H, or H2.
Unlike the case of the non-polar covalent bond forming a hydrogen molecule, discussed above, in the case of water, covalent bonds are polar covalent bonds, as the 8 protons in the oxygen nucleus has greater electrical attractive power for hydrogen's electrons than the two protons in the two hydrogen nuclei, 'polarizing' the molecule characterized by more electronegativity in the vicinity of the oxygen nucleus than in the vicinity of the hydrogen nuclei.
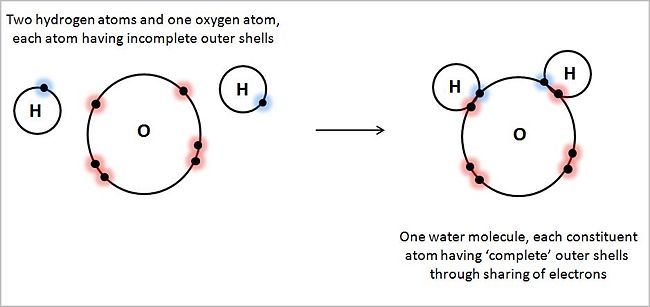
For simplicity, the illustration shows only those electrons of each atom located in its outer shell. Hydrogen atoms, with one electron each in their outer shell (1s1), behave as if they 'wanted' two electrons in their outer shell. Oxygen atoms, with six electrons in its outer shell (1s22s22p4), behave as if they 'wanted' eight electrons in their outer shell — the so-called 'octet rule'. By virtue of each hydrogen atom pairing its unpaired outer shell electron with an unpaired oxygen electron in its outer shell, sharing electrons, all three atoms approach their 'goal' of filling up their valence shells to their natural full capacity, creating in the process two covalent bonds.
How can we know that an oxygen atom has two unpaired [(↑) (↑)] electrons in its six-electron outer shell? Could it not have two paired 2s electrons (2s2 [(↑↓)] and two paired electrons each in 2px [(↑↓)] and 2py [(↑↓)] and no electrons in 2pz, as that would give it six paired electrons [(↑↓)] in its outer shell, two in the s subshell and 4 in the p subshell, in which case it would not have two unpaired electrons available to share electrons with two hydrogen atoms? The answer involves how electrons get added to orbitals in succession and to a rule called Hund’s rule. Electrons get added to orbitals in succession in a way that minimizes the total energy of the atom, as each orbital has its own energy level determined by a number of factors including the shape of the orbital (s-orbitals have lower energy than p-orbitals); the shell it finds itself in (shells more distant from the nucleus tend to have higher energy); and, the extent to which the electrons exert repulsive forces (repulsive forces between electrons raise their energy level). By Hund’s rule, for an oxygen atom, the lowest total energy level emerges when its eight electrons arrange themselves successively in the single 1s orbital, the single 2s orbital, and the three 2p orbitals, no one orbital holding more than two electrons, paired [(↑↓)]. Hund’s rule then further applies to subshells that have more than one orbital, like the p subshell in the second shell of the oxygen atom, which has three p orbitals (see Table: Features of Atomic Structure in Relation to Orbitals). Hund’s rule specifies that electrons then arrange with parallel [(↑) (↑) spins to different orbitals in the multi-orbital subshell. If it had two paired electrons each in 2px [(↑↓)] and 2py [(↑↓)] and no electrons in 2pz, the repulsive forces between the electrons in the p subshell would be greater than they would be with the electrons as specified by Hund's rule. So, to comply with Hund’s rule an oxygen atom must arrange its electrons as follows:
Thus, an oxygen atom will have two unpaired, parallel-spin electrons in its outer shell, rendering it a radical, or more specifically, a di-radical.
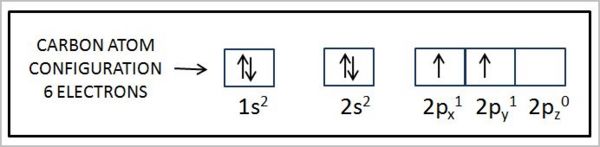
The image below shows the electron orbital configuration of another biologically important atom, carbon:
Molecular orbitals
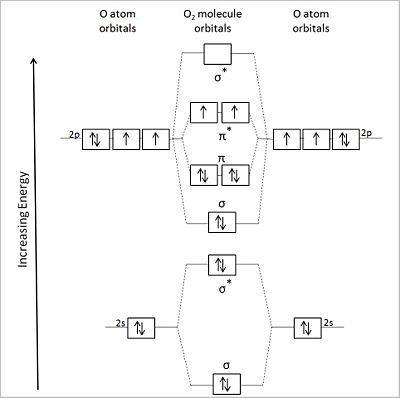
In covalently bonded atoms — a covalent molecule like the oxygen molecule, O2 — the shared electrons do not remain rigidly confined to the region of the bonded atoms of the covalent bond. Rather those shared electrons occupy newly generated orbitals that for the most part distribute throughout the molecule — referred to as ‘delocalization’ — forming so-called molecular orbitals whose properties accord with the Pauli exclusion principle, Hund's rule , and the dictates of anti-parallel spin per electron pair per orbital. Nevertheless, the electrons’ probability of localization between the atom pairs of the covalent bond continues to remain high.
The tendency of the electron pairs in a molecular covalent bond to ‘delocalize’ in the way described means that the influence of a pair of covalently bonded atoms can affect other bonds in the molecule, several bond lengths away (e.g., as when one atom in the molecule replaces another).
Molecular orbitals from the covalently bonding of two atoms acquire shapes visualizable as an overlapping of the atomic orbitals in the valence shells of the two atoms.
Because an atomic orbital has two different algebraic signs or colors, the overlapping of two atomic orbitals creates a molecular orbital that 'adds up' ('matches color'), which enhances the bonding, as well one that 'cancels out' {'color-mismatches'), weakening the bonding. The Greek letter σ designates a molecular orbital resembling an atomic s orbital, and π, one resembling a p orbital, when viewed appropriately. Both σ and π orbitals have both a bonding and antibonding component.
The accompanying figures shows a diagrammatic representation of the molecular orbitals of an oxygen molecule (center) that are formed by the overlapping valence shell orbitals of two flanking oxygen atoms (left and right of center). Asterisks designate antibonding orbitals. Note that molecular oxygen is a diradical capable of accepting four electrons to fill its valence orbitals.
Oxygen....
References cited and notes in text
Citations and Notes
- ↑ Note: Stress reactions in biological cells can result also from 'reactive species', including 'free radicals', that do not necessarily contain oxygen. The term 'reactive species' subsumes 'reactive oxygen species' (ROS) and 'oxygen free radicals'.
- ↑ Halliwell B. (2007) Biochemistry of oxidative stress. Biochemical Society Transactions. 35(part 5):1147-1150.
- ↑ Kearney, John F. Keaney; Kearney, John F., Jr. (2000) Oxidative Stress and Vascular Disease. Springer:. ISBN 0792386787, ISBN 780792386780, 373 pages.
- Author note regarding book: The purpose of this book is to provide the reader with an overview of oxidative stress as it pertains to vascular diseases. The first part of the book is designed to help the reader grapple with important issues such as: Precisely what is oxidative stress? What markers are available to assess oxidative stress? and What are the precise sources of oxidative stress and antioxidant defenses in the vasculature? With this information as background, the book goes on to address the major vascular disease syndromes for which there is good evidence that oxidative stress may be involved. The greatest body of evidence exists for atherosclerosis and, accordingly, this is the most extensive section. However, we have taken great care to try and provide the reader with objective evidence for the role of oxidative stress in other diseases such as diabetes, hypertension, and vascular remodeling. In each instance, the book is designed to provide a solid overview of the basic science data required to evaluate the role of oxidative stress. Animal data is then presented and finally, where applicable, the role of oxidative stress in human disease is discussed with the appropriate reference to epidemiologic data. The book is designed to appeal to both scientists and clinicians with an interest in vascular disease and its underlying pathophysiology.
- ↑ "aerobic" Dictionary.com; "aerobic" Online Etymology Dictionary. Douglas Harper, Historian. 30 Oct. 2008.
- ↑ Dröge W. (2002) Free Radicals in the Physiological Control of Cell Function. Physiol Rev 82:47-95. | 50 pages, 660 references.
- ↑ Electron Spin and Electron Intrinsic Angular Momentum
- Note: The notion of spin for this intrinsic property of the electron arises from thinking about the property from the classical mechanics perspective.
- ↑ J. J. Thomson Biography Biography at the Nobel Prize website
- ↑ J. J. Thomson's Nobel Prize Lecture (1906) Carriers of Negative Electricity
- "In this lecture I wish to give an account of some investigations which have led to the conclusion that the carriers of negative electricity are bodies, which I have called corpuscles, having a mass very much smaller than that of the atom of any known element, and are of the same character from whatever source the negative electricity may be derived."
- ↑ Atkins PW. (1991) Atoms, Electrons Change. Scientific American Books. New York. ISBN 978-0716750284