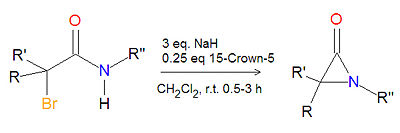Lactam: Difference between revisions
imported>Howard C. Berkowitz Tag: Reverted |
John Leach (talk | contribs) (Undo imported revision 469622 by user imported>Howard C. Berkowitz) Tag: Undo |
||
| Line 13: | Line 13: | ||
The <math>\beta</math>-lactam forms the center structure of many antibiotic drugs, such as the [[cephalosporin]]s and the [[penicillin]]s, as shown above. In the penicillins, the non-lactam ring is one atom smaller compared to the cephalosporins. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of the beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins and penicillins are often made semi-synthetically, using a core structure obtained from a natural organism, such as a fungus, due to the difficulty and expense of synthesizing these lactams. | The <math>\beta</math>-lactam forms the center structure of many antibiotic drugs, such as the [[cephalosporin]]s and the [[penicillin]]s, as shown above. In the penicillins, the non-lactam ring is one atom smaller compared to the cephalosporins. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of the beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins and penicillins are often made semi-synthetically, using a core structure obtained from a natural organism, such as a fungus, due to the difficulty and expense of synthesizing these lactams. | ||
Revision as of 16:13, 21 March 2024
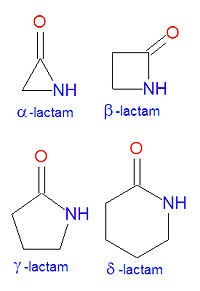
In chemistry, a lactam is a cyclic amide. The name is derived from two chemical terms, lactone, referring to a cyclic ketone, and amide, a compound containing a nitrogen atom next to a carbonyl group. Lactams are named according to the size of the cyclic ring in the lactam: -lactams, -lactams, -lactams and -lactams contain rings made of three, four, five or six atoms, respectively. -lactams are also called aziridinones. Many widely used antibiotic drugs, including the penicillins and cephalosporins, owe their activity to the presence of a -lactam structure. The lactams may have substitutions added to the nitrogen atom or any of the non-carbonyl carbon atoms in the base structure.
Synthesis of lactams
Synthesis of alpha-lactams
The cyclization reaction of an -haloamide precuror in the presence of sodium hydride and 15-crown-5 ether at room temperature in dichloromethane (CH2Cl2) is a high-yielding, general route to -lactam (aziridinone) products. The hydrogen gas and sodium halide by-products are readily removed.[1]
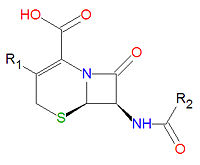
Antibiotics
The -lactam forms the center structure of many antibiotic drugs, such as the cephalosporins and the penicillins, as shown above. In the penicillins, the non-lactam ring is one atom smaller compared to the cephalosporins. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of the beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins and penicillins are often made semi-synthetically, using a core structure obtained from a natural organism, such as a fungus, due to the difficulty and expense of synthesizing these lactams.
- ↑ V. Cesare, T. M. Lyons and I. Lengyel (2002). "A High-Yielding General Synthesis of α-Lactams". Synthesis 2002: 1716-1720.