Hepatocellular cancer: Difference between revisions
imported>Robert Badgett |
imported>Robert Badgett m (→Screening) |
||
| Line 19: | Line 19: | ||
"In a mixed-aetiology cohort, the most effective surveillance strategy is to screen each patient with AFP assay and ultrasound imaging on a 6-monthly basis" according to a [[systematic review]] by the [http://www.hta.ac.uk/ NIHR Health Technology Assessment programme] (UK). <ref name="pmid17767898">{{cite journal| author=Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M et al.| title=Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. | journal=Health Technol Assess | year= 2007 | volume= 11 | issue= 34 | pages= 1-206 | pmid=17767898 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17767898 }} </ref> | "In a mixed-aetiology cohort, the most effective surveillance strategy is to screen each patient with AFP assay and ultrasound imaging on a 6-monthly basis" according to a [[systematic review]] by the [http://www.hta.ac.uk/ NIHR Health Technology Assessment programme] (UK). <ref name="pmid17767898">{{cite journal| author=Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M et al.| title=Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. | journal=Health Technol Assess | year= 2007 | volume= 11 | issue= 34 | pages= 1-206 | pmid=17767898 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17767898 }} </ref> | ||
In a [[randomized controlled trial]], the [[relative risk ratio]] of [[ | In a [[randomized controlled trial]], the [[relative risk ratio]] of biannual [[alpha-fetoprotein]] and [[ultrasonography]], as compared to [[no screening]], for mortality from hepatocellular carcinoma was 0.6 and the [[relative risk reduction]] was 36.7%. In populations similar to those in this study which had a rate of risk as measured by the mortality from hepatocellular carcinoma of 0.1315% without treatment, the [[number needed to treat]] is 2070. <ref name="pmid15042359">{{cite journal| author=Zhang BH, Yang BH, Tang ZY| title=Randomized controlled trial of screening for hepatocellular carcinoma. | journal=J Cancer Res Clin Oncol | year= 2004 | volume= 130 | issue= 7 | pages= 417-22 | pmid=15042359 | doi=10.1007/s00432-004-0552-0 | pmc= | url= }} </ref> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 07:33, 12 August 2011
Diagnosis
The alpha-fetoprotein may be elevated; however, its positive predictive value is low and it may be elevated in patients with cirrhosis.[1]
Treatment
Hepatocellular cancer treatment information from the National Cancer Institute's Physician Data Query
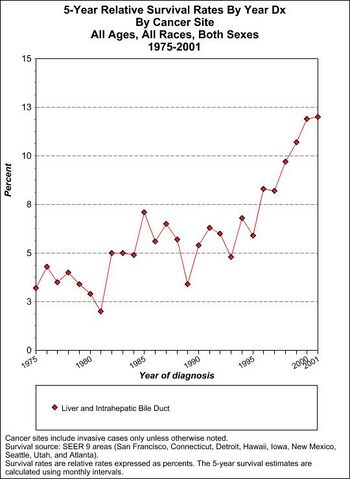
Prognosis
Staging information
Hepatocellular cancer staging information from the National Cancer Institute's Physician Data Query
Screening
"Surveillance is deemed cost-effective if the expected HCC risk exceeds 1.5% per year in patients with hepatitis C and 0.2% per year in patients with hepatitis B. Analysis of recent studies show that alpha-fetoprotein determination lacks adequate sensitivity and specificity for effective surveillance (and for diagnosis). Thus, surveillance has to be based on ultrasound examination. The recommended screening interval is 6 months" according to a clinical practice guideline by the American Association for the Study of Liver Diseases. [2]
"In a mixed-aetiology cohort, the most effective surveillance strategy is to screen each patient with AFP assay and ultrasound imaging on a 6-monthly basis" according to a systematic review by the NIHR Health Technology Assessment programme (UK). [3]
In a randomized controlled trial, the relative risk ratio of biannual alpha-fetoprotein and ultrasonography, as compared to no screening, for mortality from hepatocellular carcinoma was 0.6 and the relative risk reduction was 36.7%. In populations similar to those in this study which had a rate of risk as measured by the mortality from hepatocellular carcinoma of 0.1315% without treatment, the number needed to treat is 2070. [4]
References
- ↑ Tong MJ, Blatt LM, Kao VW (2001). "Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America.". J Gastroenterol Hepatol 16 (5): 553-9. PMID 11350553.
- ↑ Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011). "Management of hepatocellular carcinoma: an update.". Hepatology 53 (3): 1020-2. DOI:10.1002/hep.24199. PMID 21374666. PMC PMC3084991. Research Blogging.
- ↑ Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M et al. (2007). "Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.". Health Technol Assess 11 (34): 1-206. PMID 17767898. [e]
- ↑ Zhang BH, Yang BH, Tang ZY (2004). "Randomized controlled trial of screening for hepatocellular carcinoma.". J Cancer Res Clin Oncol 130 (7): 417-22. DOI:10.1007/s00432-004-0552-0. PMID 15042359. Research Blogging.