Staphylococcus epidermidis: Difference between revisions
imported>Sarah M. Maurice |
imported>Sarah M. Maurice |
||
| Line 29: | Line 29: | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
As a gram-positive bacterium, ''S. epidermidis'' has a cell wall made of a large concentration of peptidoglycan layer but no outer membrane. The cell-wall anchored(CWA)proteins of these bacteria are part of a family of surface-exposed proteins that interact with | As a gram-positive bacterium, ''S. epidermidis'' has a cell wall made of a large concentration of peptidoglycan layer but no outer membrane. The cell-wall anchored(CWA)proteins of these bacteria are part of a family of surface-exposed proteins that interact with targets in the host. These CWA proteins possess an N-terminal secretion signal sequence (S) required for sec-dependent secretion, followed by a non-repeat A domain that contains the ligand-binding site. These interactions are particular important because they are involved in bacterial adherence and escape from the host defense systems. No mechanism is known to explain the virulence of S. epidermidis, but it is thought that the cell wall proteins are potential factors that must be involved. | ||
==Ecology== | ==Ecology== | ||
Revision as of 21:35, 21 April 2009
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Staphylococcus epidermidis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Staphylococcus epidermidis |
Description and significance
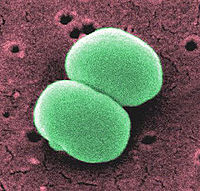
Staphylococcus epidermidis is a gram positive coccus, a normal inhabitant of the human skin that grows in clusters. It is an aerobic organism but is yet capable of growing anaerobically when placed in a standardized, complex medium of glucose. Research studies reveal that S. epidermidis lives in close association with S. aureus, a very destructive pathogen.
Even though a coagulase-negative gram bacterium, S. epidermidis has been lately classified among the most important pathogens responsible for divers nosocomial infections. Most strains are highly resistant to multiple antibiotics, such as penicillin, tetracycline, methicillin and many more, which makes it very difficult to treat the infections resulting from these bacteria. According to the Centers for Disease Control and Prevention's National Nosocomial infection surveillance system, S. epidermidis is responsible for 33.5% of nosocomial blood stream infections.
Genome structure
Among all the S. epidermidis strains, the genome sequence of only 2 strains has been completely described: S. epidermidis RP62a with a genome length of 2,616,530 bp and S. epidermids ATCC12228, 2,499,279 bp. RP62a encodes 2585 protein genes, 61 tRNA and 19 rRNA; whereas the ATCC12228 strain contains a number of 2381 protein coding genes, 60tRNA and 16rRNA. The low G+C content (32.1% for both strains) stands for the virulence and the high resistance of S. epidermidis. The genomic elements, which include, the genome islands, the insertions sequences, the composite transposons and the integrated plasmids, constitute more or less 9% of the genome.
Cell structure and metabolism
As a gram-positive bacterium, S. epidermidis has a cell wall made of a large concentration of peptidoglycan layer but no outer membrane. The cell-wall anchored(CWA)proteins of these bacteria are part of a family of surface-exposed proteins that interact with targets in the host. These CWA proteins possess an N-terminal secretion signal sequence (S) required for sec-dependent secretion, followed by a non-repeat A domain that contains the ligand-binding site. These interactions are particular important because they are involved in bacterial adherence and escape from the host defense systems. No mechanism is known to explain the virulence of S. epidermidis, but it is thought that the cell wall proteins are potential factors that must be involved.
Ecology
Pathology
Most infections caused by S. epidermidis affect hospital patients because these organisms develop structures called biofilms on the surface of implanted medical devices. These biofilms result from the agglomeration of bacterial cells that are contained in an exopolysaccharide matrix, the slime, to produce a very dense and protected environment from the host defense systems and antibiotics. They not only provide protection from the host but they also result in the damage of surrounding tissues of the host.
Skin and tissues infections, post-surgical wounds are the common points of entry for infection by S. epidermis. This bacterium accounts for most catheter-related infections, joint replacement infections, the majority of prosthetic cardiac valve infections and infections following post-neurosurgical procedures.In addition, studies have reported that S. epidermidis is highly predominant in the milk and feces of breast-fed infants compared to formula-fed ones. However, whether or not the presence of these bacteria can cause infections in these children have not been determined.
Moreover, S. epidermis plays a significant role in some external ocular diseases, such as chronic blepharitis and suppurative keratitis. Fortunately, these infections are treatable; and ciprofloxacin is considered the best drug in the cure of bacterial keratitis. Interestingly, methicillin-resistant S. epidermidis was identified in the cause of nosocomial meningitis when prosthetic devices were used in a trauma case in 2003. Even though, the antibiotic, vancomycin, was the treatment of choice, it was not successful in the recovery of the patient. Instead, better results were obtained with the use of linezolid. Due to their high resistance to antimicrobial agents, other techniques have been employed to prevent the staphylococcal infections which imply mechanical cleaning, the stopping of biofilm growth by the removal of essential nutrients, the inhibition of microbial attachments to surfaces, and biomass detachment.