Michael Faraday: Difference between revisions
imported>Paul Wormer |
imported>Richard Jensen (add articles) |
||
| Line 41: | Line 41: | ||
* Bence Jones, Henry. ''The Life and Letters of Faraday,'' (2 vol. 1870, reprint 2006) [http://books.google.com/books?id=9C0DAAAAYAAJ&printsec=frontcover&dq=intitle:faraday+inauthor:Bence+inauthor:Jones&lr=&num=30&as_brr=0 online first edition] | * Bence Jones, Henry. ''The Life and Letters of Faraday,'' (2 vol. 1870, reprint 2006) [http://books.google.com/books?id=9C0DAAAAYAAJ&printsec=frontcover&dq=intitle:faraday+inauthor:Bence+inauthor:Jones&lr=&num=30&as_brr=0 online first edition] | ||
* Cantor, Geoffrey. ''Michael Faraday: Sandemanian and Scientist.'' (1991) | * Cantor, Geoffrey. ''Michael Faraday: Sandemanian and Scientist.'' (1991) | ||
* Gribbin, J. ''The Scientists, A History of Science Told Through the Lives of Its Greatest Inventors'', Random House, New York, 2002. Paperback edition (2004) pp.412 - 424. | * Gribbin, J. ''The Scientists, A History of Science Told Through the Lives of Its Greatest Inventors'', Random House, New York, 2002. Paperback edition (2004) pp. 412-424. | ||
* Jensen, William B. "Michael Faraday and the Art and Science of Chemical Manipulation." ''Bulletin for the History of Chemistry'' (1991) (No. 11): 65–76 | |||
* Romo, Jose, and Manuel Doncel. "Faraday's initial mistake concerning the direction of induced currents." ''Archive for History of Exact Sciences'' 1994. 47: 291–385. | * Romo, Jose, and Manuel Doncel. "Faraday's initial mistake concerning the direction of induced currents." ''Archive for History of Exact Sciences'' 1994. 47: 291–385. | ||
* Steinle, Friedrich. "Work, Finish, Publish? The formation of the second series of Faraday's 'Experimental researches in electricity.'" ''Physis'', 1996. 33: 141–220. | * Steinle, Friedrich. "Work, Finish, Publish? The formation of the second series of Faraday's 'Experimental researches in electricity.'" ''Physis'', 1996. 33: 141–220. | ||
* Tweney, Ryan D. . "Faraday's notebooks: The active organization of creative science." ''Physics Education''. 1991 26: 301–306. | * Tweney, Ryan D. . "Faraday's notebooks: The active organization of creative science." ''Physics Education''. 1991 26: 301–306. | ||
* Tweney, Ryan D. "Discovering Discovery: How Faraday Found the First Metallic Colloid," ''Perspectives on Science'' Volume 14, Number 1, Spring 2006, pp. 97-121 in [[Project Muse]] | |||
* Tweney, Ryan D. "Stopping Time: Faraday and the Scientific Creation of Perceptual Order." ''Physis'' 1992. 29: 149–164. | |||
===Primary sources=== | ===Primary sources=== | ||
| Line 52: | Line 54: | ||
* Faraday, Michael. '' Experimental Researches in Chemistry and Physics,'' (1859) 496 pages; [http://books.google.com/books?id=AUwNAAAAYAAJ&printsec=frontcover&dq=inauthor:Faraday+inauthor:Michael&lr=&num=30&as_brr=0 online edition] | * Faraday, Michael. '' Experimental Researches in Chemistry and Physics,'' (1859) 496 pages; [http://books.google.com/books?id=AUwNAAAAYAAJ&printsec=frontcover&dq=inauthor:Faraday+inauthor:Michael&lr=&num=30&as_brr=0 online edition] | ||
* Faraday, Michael. ''The Correspondence of Michael Faraday.'' edited by Frank A.J.L. James. Volume 1: 1811—1831; Volume 2: 1832–1840; Volume 3: 1841–1848. Volume 4: 1849–1855 (1991–1996) | * Faraday, Michael. ''The Correspondence of Michael Faraday.'' edited by Frank A.J.L. James. Volume 1: 1811—1831; Volume 2: 1832–1840; Volume 3: 1841–1848. Volume 4: 1849–1855 (1991–1996) | ||
* Martin, Thomas, ed. ''Faraday's Diary: Being the Various Philosophical Notes of Experimental Investigation Made by Michael Faraday During the Years 1820–1862'' (7 vol 1932-36). | |||
====notes==== | ====notes==== | ||
<references /> | <references /> | ||
Revision as of 16:52, 8 April 2008
Michael Faraday (22 September 1791 – 25 August 1867) was an English physicist and chemist. In 1821, soon after Oersted's discovery, he published his work on—what he called—electromagnetic rotation (the principle behind the electric motor). In 1831, Faraday discovered electromagnetic induction, the principle behind the electric transformer and electric generator. In chemistry Faraday discovered benzene, contributed importantly to electrochemistry, and popularized terminology such as anode, cathode, electrode, and ion.
Biography
The Faraday family came from the North of England. Before Michael was born, his father, a blacksmith, took his wife and two small children south, in search of work. The family settled briefly in Newington Butts, which was then a separate village but is now part of Southwark a borough in South London, where Michael was born. Soon the family moved into London itself. Michael's father had poor health (he died in 1810) and Michael grew up in poverty. The family was close and obtained strength from their orthodox faith, Sandemanian, a splitoff from the Presbyterian church. Michael would stay faithful to his religion for the rest of his life.
At fourteen Michael became an apprentice at a bookseller and bookbinder's shop and moved in with his boss's family. In this household were plenty of books around for Michael to read. For instance the third edition of Encyclopedia Brittanica was one of his bookbinding assignments. His fascination for electricity was first stirred by reading the encyclopedia article about it. From a customer of the shop Faraday, twenty-one and nearing the end of his apprenticeship, received tickets for a series of four lectures on chemistry delivered by Sir Humphry Davy at the Royal Institution. These lectures, in the spring of 1812, were recorded by Faraday in careful lecture notes and neatly bound by him as a book.
In the fall of 1812 Michael was a fully-qualified bookbinder and hired by another firm. Faraday didn't particularly like his new job, but within a few weeks his life took a sudden turn. Davy needed help for a few days from somebody with a little knowledge of chemistry and Faraday got the job, probably by a good word from another customer of his earlier boss. Faraday showed his lecture notes to Davy, who must have been pleased by so much attention to his words. After the temporary job ended, Faraday had to leave the Royal Institution as there was no money to continue his position. However, as luck would have it, not much later Humphry Davy's laboratory assistant was fired because of a drinking problem and his position became vacant. Not surprisingly, Faraday got the job and started on 1 March 1813 at the Royal Institution where he would stay for fifty-two years, until 1865.
Half a year after obtaining this position, Faraday was invited by Sir Davy to accompany him and Lady Davy on a tour of Europe. Davy was a renowned "natural philosopher" (later the name "scientist" was coined for this profession by Whewell) and had entry to many scientific circles on the continent. Michael accepted the invitation, but during the tour sometimes regretted this, because Lady Davy, who is known to have been very snobbish, treated him as a lowly servant. Professionally the tour was a great success for Faraday, though, because he had the chance to converse with many of the leading scientists in France, Switzerland and Italy. He saw he Alps and the Mediterranean, learned French and Italian, and became Davy's collaborator, not just his assistant. The chemist John Hall Gladstone (1827–1902), who knew Faraday well, wrote:[1]
This year and a half may be considered as the time of Faraday's education; it was the period of his life that best corresponds with the collegiate course of other men who have attained high distinction in the world of thought. But his University was Europe; his professors the master whom he served, and those illustrious men to whom the renown of Davy introduced the travelers".
(To be continued)
Faraday's science
Electromagnetic rotation
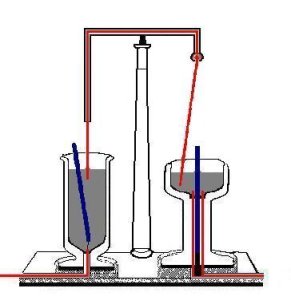
When Faraday heard of Oersted's discovery (1820) that a steady electric current in a wire generates a circular magnetic field (with the current-carrying wire the center of the field), it occurred to him that a magnetic pole would be pushed around a circle by such a field and hence would rotate forever, or at least as long as the current is running. In 1821 he designed the apparatus shown in the figure on the right.[2] In the vessel on the left a strong bar magnet floats in mercury held in place only by a thread at its bottom. Recall that mercury is a heavy liquid that is a very good conductor of electricity. A fixed cupper wire is dipped into the mercury. At the bottom of the vessel a wire sticks into the mercury. In the vessel on the right a bar magnet is fixed in a conducting (copper) socket that extends into the bath and a copper wire that hangs from a kind of ball bearing is dipped into the mercury. When a direct current is switched on (running through the wires and the mercury baths) the wire on the right rotates around the magnet so fast that—as described by Faraday—the eye can scarcely follow the motion. The magnet on the left rotates around the fixed wire. Note that Faraday's setup is such that only one pole of the two poles of the magnets is involved. If the magnets are turned around (i.e., the North and South poles are interchanged) the rotation of the wire and the magnet changes direction. The same happens if the current runs in opposite direction. Faraday, who coined the term electromagnetic rotation for this effect, had in fact invented the electric motor.
Magnetic induction
Chemistry
To the displeasure of his boss Humphry Davy, Faraday managed to liquify chlorine in 1823. Davy felt that he deserved credit for this discovery, since he had suggested the problem to his coworker. It is likely that this feeling of displeasure was one of the reasons that Davy later objected to Faraday becoming fellow of the Royal Society. In 1825 Faraday discovered benzene, which he called bicarburet of hydrogen. He isolated it from a liquid obtained in the production of oil gas. At the same time he did research on glass that, however, did not have much result; but he found the recipe for heavy glass. Around 1845 Faraday discovered that the polarization of light by different kinds of glass, among which heavy glass, is affected by a magnetic field. This rotation of the polarization plane of light by a magnetic field, is now known as the Faraday effect.
In the 1830s Faraday did very important work in electrochemistry and formulated two laws that now carry his name. The basic setup of electrochemistry is simple: it consists of a vessel containing an electrolyte, which is a solution of charged particles (ions). Since this solution is neutral in total, the total charge carried by the positive particles (cations) is he same (in absolute value) as the total charge carried by the negative particles (anions). Two electrodes are dipped into the electrolyte. Outside the vessel a direct current (DC) runs from the negatively charged electrode (the cathode) to the positively charged electrode (the anode). Inside the vessel cations move to the cathode, pick up electrons—which carry negative charge—so that the cations are neutralized and are deposited on the cathode (or if the neutral species is gaseous, it escapes from the cathode in the form of gas). At the anode the opposite happens: anions lose their excess electrons and are deposited. Outside the vessel the electrons run from the anode to the cathode (by definition they run in direction opposite to the DC). Faraday's first law states that the amount of substance deposited on the electrodes is proportional to the amount of current that passed through the electrodes. In the case of a steady current this amount is equal to the time that the current has been running multiplied by the strength of the current. A constant proportional to this amount is called after Faraday—Faraday's constant. Faraday's second law of electrochemistry is a recognition of the fact that anions and cations may carry one, two, three, etc. elementary charges, so that it takes one, two, three, etc., electrons, respectively, to neutralize the cation, while the anion must lose one, two, three, etc. electron to become neutral. These differently charged ions will all give the deposition of one atom only, so that, e.g., twice as much electricity is needed to generate an atom from a doubly charged ion as from a singly charged ion. Faraday was the first to see these facts clearly, moreover, he introduced the corresponding terminology (electrolyte, electrode, anode, cathode, ion, anion, and cation), helped by William Whewell of Trinity College, Cambridge, who knew Greek and Latin.
Bibliography
- Anderson, Ronald. "The Crafting of Scientific Meaning and Identity: Exploring the Performative Dimensions of Michael Faraday's Texts," Perspectives on Science" - Volume 14, Number 1, Spring 2006, pp. 7-39 in Project Muse
- Bence Jones, Henry. The Life and Letters of Faraday, (2 vol. 1870, reprint 2006) online first edition
- Cantor, Geoffrey. Michael Faraday: Sandemanian and Scientist. (1991)
- Gribbin, J. The Scientists, A History of Science Told Through the Lives of Its Greatest Inventors, Random House, New York, 2002. Paperback edition (2004) pp. 412-424.
- Jensen, William B. "Michael Faraday and the Art and Science of Chemical Manipulation." Bulletin for the History of Chemistry (1991) (No. 11): 65–76
- Romo, Jose, and Manuel Doncel. "Faraday's initial mistake concerning the direction of induced currents." Archive for History of Exact Sciences 1994. 47: 291–385.
- Steinle, Friedrich. "Work, Finish, Publish? The formation of the second series of Faraday's 'Experimental researches in electricity.'" Physis, 1996. 33: 141–220.
- Tweney, Ryan D. . "Faraday's notebooks: The active organization of creative science." Physics Education. 1991 26: 301–306.
- Tweney, Ryan D. "Discovering Discovery: How Faraday Found the First Metallic Colloid," Perspectives on Science Volume 14, Number 1, Spring 2006, pp. 97-121 in Project Muse
- Tweney, Ryan D. "Stopping Time: Faraday and the Scientific Creation of Perceptual Order." Physis 1992. 29: 149–164.
Primary sources
- Faraday, Michael. Experimental Researches in Electricity (1855) online edition
- Faraday, Michael. The Chemical History Of A Candle (1908) online edition
- Faraday, Michael. Experimental Researches in Chemistry and Physics, (1859) 496 pages; online edition
- Faraday, Michael. The Correspondence of Michael Faraday. edited by Frank A.J.L. James. Volume 1: 1811—1831; Volume 2: 1832–1840; Volume 3: 1841–1848. Volume 4: 1849–1855 (1991–1996)
- Martin, Thomas, ed. Faraday's Diary: Being the Various Philosophical Notes of Experimental Investigation Made by Michael Faraday During the Years 1820–1862 (7 vol 1932-36).
notes
- ↑ J.H. Gladstone, Michael Faraday, Harper & Brothers, New York (1872). [Online: http://home.att.net/~l.caimi/FARADAY-1.doc]
- ↑ M. Faraday, Experimental researches in electricity, vol II, Richard and John Edward Taylor, London (1844). Plate IV On line