Piperacillin: Difference between revisions
imported>David E. Volk (Chem infobox + capitalize Gram- +/-) |
imported>David E. Volk mNo edit summary |
||
| Line 4: | Line 4: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
|image=[[Image:Piperacillin structure.jpg| | |image=[[Image:Piperacillin structure.jpg|center|thumb|250px|{{#ifexist:Template:Piperacillin structure.jpg/credit|{{Piperacillin structure.jpg/credit}}<br/>|}}]] | ||
|width=250px | |width=250px | ||
|molname=piperacillin | |molname=piperacillin | ||
Revision as of 12:24, 3 April 2008
|
| |||||||
| piperacillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | penicillin-like | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
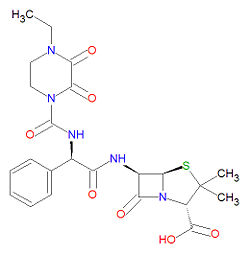
Piperacillin, marketed as Pipracil®, is a beta-lactam antibiotic drug derived from ampicillin that is similar to penicillin and its other derivatives. It has activity against both Gram-negative and Gram-positive aerobic and anaerobic bacteria.
Mechanism of action
Piperacillin, like other penicillin drugs, functions by binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, thereby inhibiting the final stage of cell wall synthesis and leading to autolysis of the bacteria by autolysins.
Chemistry
Piperacillin is a -lactam. Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has chemical formula C23H27N5O7S (MW = 517.5548 g/mol). It is subject to degradation in bacteria which express beta-lactamase.
Drug interactions
The anticoagulant effects of acenocoumarol, anisindione, dicumarol and warfarin are increased when taken with piperacillin. Piperacillin also increases the effects of some muscle relaxants, including atracurium, doxacurium, gallamine triethiodide, metocurine, mivacurium, pancuronium, pipecuronium, rocuronium, succinylcholine, tubocurarine, and vecuronium. The effectiveness of the contraceptives ethinyl estradiol and mestranol are decreased when taken with piperacillin and many other penicillin derivatives. Tetracycline and its derivatives demecloclycine, doxycycline, methacycline, minocycline, oxytetracycline and rolitetracycline may be antogists of this drug. The effects and toxicity of methotrexate increases when taken with piperacillin.[1]
External links
- Piperacillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank