Crystal: Difference between revisions
imported>Nereo Preto (all minerals are crystallized per definition) |
imported>Anthony Argyriou (expand) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
A '''crystal''' | A '''crystal''' is a [[solid]] in which the constituent atoms are arranged in an orderly, repeating pattern, as distinct from the arrangement of atoms in an [[amorphous solid]]. The repeating polyhedral pattern of atoms creates preferred [[cleavage]] planes, and affects the mechanical and optical properties of the material. | ||
The structure of a crystal depends on the size and chemical properties of the constituent atoms, and can take many different forms. These forms are categorized in several ways, depending on the geometry or the chemistry of the structures. The most basic forms are cubic and hexagonal, but many variations of these structures and many other structures also exist. | |||
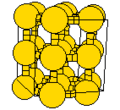
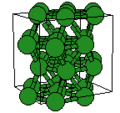
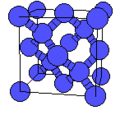
<gallery caption="A sample of common crystal structures at the atomic level"> | |||
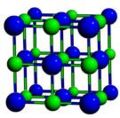
Image:NaCl-b1.jpg|Cubic crystal structure of NaCl | |||
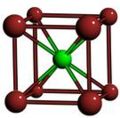
Image:CsCl-BCC-b2.jpg|Body-centered cubic crystal structure of CsCl | |||
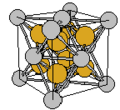
Image:Cu3Au-FCC-l1 2.jpg|Face-centered cubic crystal structure of Cu<sub>3</sub>Au | |||
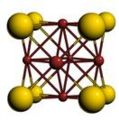
Image:CaF2-c1.png|Cubic structure of CaF<sub>2</sub>, fluorite | |||
Image:SimpleHexagonalLattice-a f.png|Simple hexagonal lattice structure | |||
Image:Mg-HCP-a3.png|Hexagonal close-packed crystal structure of Mg | |||
Image:Diamond-a4.png|Crystal structure of [[diamond]] | |||
Image:Graphite-a9.png|Crystal structure of [[graphite]] | |||
</gallery> | |||
Crystal structures are not always perfect in nature, as defects can form in the structure due to impurities or stresses on the crystal. These impurities can have important effects on the mechanical and electrical properties of the crystal. | |||
Revision as of 12:36, 7 February 2008
A crystal is a solid in which the constituent atoms are arranged in an orderly, repeating pattern, as distinct from the arrangement of atoms in an amorphous solid. The repeating polyhedral pattern of atoms creates preferred cleavage planes, and affects the mechanical and optical properties of the material.
The structure of a crystal depends on the size and chemical properties of the constituent atoms, and can take many different forms. These forms are categorized in several ways, depending on the geometry or the chemistry of the structures. The most basic forms are cubic and hexagonal, but many variations of these structures and many other structures also exist.
- A sample of common crystal structures at the atomic level
Crystal structure of diamond
Crystal structure of graphite
Crystal structures are not always perfect in nature, as defects can form in the structure due to impurities or stresses on the crystal. These impurities can have important effects on the mechanical and electrical properties of the crystal.