Ester: Difference between revisions
imported>Ro Thorpe |
imported>Simon Overduin m (added some more synthesis information) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
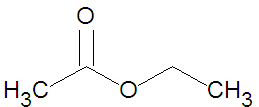
[[Image:Ethyl Acetate stickfigure.jpg|right|thumb|350px|{{#ifexist:Template:Ethyl Acetate stickfigure.jpg/credit|{{Ethyl Acetate stickfigure.jpg/credit}}<br/>|}}Ethyl acetate, a small ester.]] | [[Image:Ethyl Acetate stickfigure.jpg|right|thumb|350px|{{#ifexist:Template:Ethyl Acetate stickfigure.jpg/credit|{{Ethyl Acetate stickfigure.jpg/credit}}<br/>|}}Ethyl acetate, a small ester.]] | ||
In chemistry, an '''ester''' is a chemical compound that contains a carbonyl functionality attached to an alkoxide | In chemistry, an '''ester''' is a chemical compound that contains a carbonyl functionality attached to an alkoxide. | ||
== | Esters are widely used in the food industry as artificial flavors. Small esters can be used as solvents. | ||
==Synthesis== | |||
Esters may be formed through a variety of [[esterification]] reactions, most typically the condensation reaction of a [[carboxylic acid]] and an [[alcohol]]<ref name=McMurry1996>McMurry, J. (1996). Organic Chemistry (4th ed.). Scarborough, Ontario: Nelson Canada. pp. 222, 639, 803, 814, 816-817, 823-825.</ref>. For instance, the ester ethyl acetate (shown in the figure) can be produced from the condensation reaction between [[acetic acid]] and [[ethanol]]. A second method of ester preparation ([[alcoholyses]]) uses an acid chloride instead of a carboxylic acid. | |||
Both methods involve [[nucleophilic acyl substitution]], that is, the replacement of one [[nucleophile]] attached to a [[carbonyl]] group with a superior nucleophile<ref name=McMurry1996 />. In alcoholyses, the leaving nucleophile is a [[halide]] rather than a [[hydroxyl]] group. | |||
==References== | |||
<references/> | |||
Revision as of 16:17, 5 March 2008
In chemistry, an ester is a chemical compound that contains a carbonyl functionality attached to an alkoxide.
Esters are widely used in the food industry as artificial flavors. Small esters can be used as solvents.
Synthesis
Esters may be formed through a variety of esterification reactions, most typically the condensation reaction of a carboxylic acid and an alcohol[1]. For instance, the ester ethyl acetate (shown in the figure) can be produced from the condensation reaction between acetic acid and ethanol. A second method of ester preparation (alcoholyses) uses an acid chloride instead of a carboxylic acid.
Both methods involve nucleophilic acyl substitution, that is, the replacement of one nucleophile attached to a carbonyl group with a superior nucleophile[1]. In alcoholyses, the leaving nucleophile is a halide rather than a hydroxyl group.