Benzene: Difference between revisions
Jump to navigation
Jump to search

imported>Howard C. Berkowitz No edit summary |
imported>Caesar Schinas m (Bot: Update image code) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
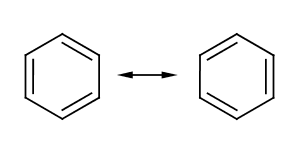
{{Image|Chemistry - Benzene - Kekule Structure.png|right|350px|The commonly recognized, but somewhat inaccurate Kekulé representation of Benzene, which Kekulé said came to him in a dream}} | |||
'''Benzene''' (C<sub>6</sub>H<sub>6</sub>) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics. | '''Benzene''' (C<sub>6</sub>H<sub>6</sub>) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics. | ||
Revision as of 05:22, 8 June 2009
Benzene (C6H6) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics.