Estrogen: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (typos) |
imported>David E. Volk No edit summary |
||
| Line 3: | Line 3: | ||
An '''estrogen''' is a type of [[steroid]] [[hormone]] with eighteen carbons. With increasing age and menopause, the levels of estrogens decrease in women, and [[estrogen replacement therapy]] has been used for decades to decrease the systoms associated with menopause. However, recent studies suggest that estrogen replacement therapy increases the risks of heart attacks and strokes, so its use is now declining. The steroids [[estrone]] and [[estradiol]] are both estrogens. | An '''estrogen''' is a type of [[steroid]] [[hormone]] with eighteen carbons. With increasing age and menopause, the levels of estrogens decrease in women, and [[estrogen replacement therapy]] has been used for decades to decrease the systoms associated with menopause. However, recent studies suggest that estrogen replacement therapy increases the risks of heart attacks and strokes, so its use is now declining. The steroids [[estrone]] and [[estradiol]] are both estrogens. | ||
== biosynthesis == | |||
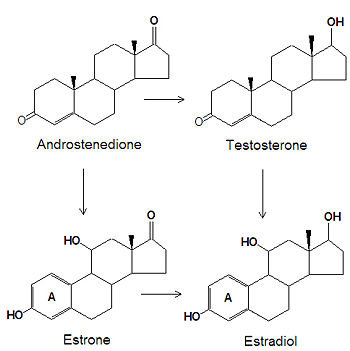
The estrogens are biosynthesized, by several chemical modifications of androgens. The C-3 ketone group is reduced to an alcohol, the C-19 methyl group is removed and the "A" ring (see [[steroid]] for numbering and nomenclature) becomes an [[aromatic]] ring. | |||
== estrogen replacement therapy (ERT) == | == estrogen replacement therapy (ERT) == | ||
Revision as of 16:20, 24 December 2007
An estrogen is a type of steroid hormone with eighteen carbons. With increasing age and menopause, the levels of estrogens decrease in women, and estrogen replacement therapy has been used for decades to decrease the systoms associated with menopause. However, recent studies suggest that estrogen replacement therapy increases the risks of heart attacks and strokes, so its use is now declining. The steroids estrone and estradiol are both estrogens.
biosynthesis
The estrogens are biosynthesized, by several chemical modifications of androgens. The C-3 ketone group is reduced to an alcohol, the C-19 methyl group is removed and the "A" ring (see steroid for numbering and nomenclature) becomes an aromatic ring.