Cysteine: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk No edit summary |
||
| Line 6: | Line 6: | ||
==Biosynthesis== | ==Biosynthesis== | ||
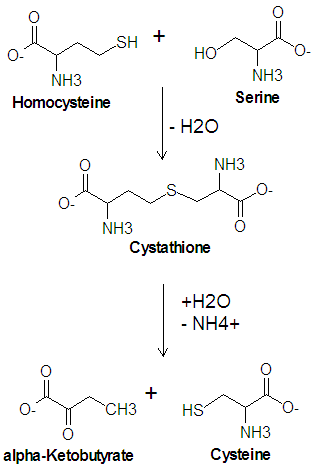
The enzyme [[cystathionine synthase]] catalyzes the condensation reaction of serine with homocysteine which produces cystathione and water. Another enzyme, [[cystathionase]], then catalyzes the deamination and cleavage of cystathione to produce cysteine and [[alpha-ketobutyrate|<math>\alpha</math>-ketobutyrate]]. In this cleavage reaction, serine acts as the carbon skeleton and homocysteine provides the sulfur atom. | The enzyme [[cystathionine synthase]] catalyzes the condensation reaction of serine with homocysteine which produces cystathione and water. Another enzyme, [[cystathionase]], then catalyzes the deamination and cleavage of cystathione to produce cysteine and [[alpha-ketobutyrate|<math>\alpha</math>-ketobutyrate]]. In this cleavage reaction, serine acts as the carbon skeleton and homocysteine provides the sulfur atom. | ||
==Disulfide bonds== | |||
Cysteine can form disulfide bonds by oxidation, and when two cysteine molecules react to form a double bond, the result is [[cystine]]. Disulfide bonds can also occur between two cysteine amino acids in a protein, and such disulfide bonds add to the stability of many proteins and keeps them folded. In general, intracellular proteins tend not to have disulfide bonds while extracellular proteins tend to have several disulfide bonds. | |||
Revision as of 18:05, 19 December 2007
Cysteine is one of the twenty common amino acids. It is one of two amino acids which contain a sulfur atom, the other being methionine, and is one of the two amino acids which contain a hydroxyl group, the other being threonine. Cysteine is a precursor of methionine in the activated methyl cycle, and it is synthesized from a condensation reaction between the amino acid serine and homocysteine.
Biosynthesis
The enzyme cystathionine synthase catalyzes the condensation reaction of serine with homocysteine which produces cystathione and water. Another enzyme, cystathionase, then catalyzes the deamination and cleavage of cystathione to produce cysteine and -ketobutyrate. In this cleavage reaction, serine acts as the carbon skeleton and homocysteine provides the sulfur atom.
Disulfide bonds
Cysteine can form disulfide bonds by oxidation, and when two cysteine molecules react to form a double bond, the result is cystine. Disulfide bonds can also occur between two cysteine amino acids in a protein, and such disulfide bonds add to the stability of many proteins and keeps them folded. In general, intracellular proteins tend not to have disulfide bonds while extracellular proteins tend to have several disulfide bonds.