Contraception (medical methods): Difference between revisions
Pat Palmer (talk | contribs) |
Pat Palmer (talk | contribs) (→Induced abortion: U.S. supreme court has unanimously ruled to keep mifepristone available) |
||
| Line 132: | Line 132: | ||
Two pharmaceuticals used in combination are necessary for this often-preferred medical method of terminating a pregnancy within three months of impregnation. The first drug, mifepristone, blocks the reproductive hormone progesterone, and the second, misoprostol, is taken one or two days later to prompt contractions and help the uterus expel its contents.<ref name=AbPill /> As of 2023, more than half of all pregnancies terminations in the U.S. are done using these drugs rather than by surgical methods. The Cleveland Clinic has a web page which provides a complete description of how these pharmaceuticals can be used, including procedural details, risks and benefits, recovery and outlook, and when to call the doctor.<ref name=ClevelandClinic /> The two drugs have been available for combined use since 2000, but in 2016, the [[United States of America|U.S.]] [[Food and Drug Administration]] (FDA), deeming the two drugs proven safe by hundreds of medical studies and long use, took a series of steps to increase the availability of distribution of mifepristone. In 2023, this means that, in states where terminating a pregnancy is legal, people can obtain the two prescription drugs at a local drug store or by a mail-order, similar to any other medicine.<ref name=Availability /> Women may take them at home, while remaining in touch with a nurse or doctor via phone or internet throughout the process<ref name=PPonAbortPill />, and thus avoid the risk of harrassment and threats of violence that have frequently been encountered by women who visit a birth control clinic and also, sometimes, to avoid involving family members or friends who might raise objections. As such, abortion opponents were particularly incensed that these drugs might make it easier for a woman to terminate a pregnancy without their family knowing about it. | Two pharmaceuticals used in combination are necessary for this often-preferred medical method of terminating a pregnancy within three months of impregnation. The first drug, mifepristone, blocks the reproductive hormone progesterone, and the second, misoprostol, is taken one or two days later to prompt contractions and help the uterus expel its contents.<ref name=AbPill /> As of 2023, more than half of all pregnancies terminations in the U.S. are done using these drugs rather than by surgical methods. The Cleveland Clinic has a web page which provides a complete description of how these pharmaceuticals can be used, including procedural details, risks and benefits, recovery and outlook, and when to call the doctor.<ref name=ClevelandClinic /> The two drugs have been available for combined use since 2000, but in 2016, the [[United States of America|U.S.]] [[Food and Drug Administration]] (FDA), deeming the two drugs proven safe by hundreds of medical studies and long use, took a series of steps to increase the availability of distribution of mifepristone. In 2023, this means that, in states where terminating a pregnancy is legal, people can obtain the two prescription drugs at a local drug store or by a mail-order, similar to any other medicine.<ref name=Availability /> Women may take them at home, while remaining in touch with a nurse or doctor via phone or internet throughout the process<ref name=PPonAbortPill />, and thus avoid the risk of harrassment and threats of violence that have frequently been encountered by women who visit a birth control clinic and also, sometimes, to avoid involving family members or friends who might raise objections. As such, abortion opponents were particularly incensed that these drugs might make it easier for a woman to terminate a pregnancy without their family knowing about it. | ||
In 2023, a lawsuit brought before a Texas judge (a state that is vehemently against pregnancy terminations) resulted in a ruling that would make the drug mifepristone illegal in all of the U.S. by classifying mifepristone as unsafe. The decision was immediately appealed, and stays against banning the drug were put in place. Removing mifepristone from the market would mean that medically induced abortion, the safest, most convenient and most affordable method of pregnancy termination, would be unavailable to anyone. The claim was made that the FDA had made mifepristone available without proof that it is safe, and that the court, which is not made of medical experts, should overrule a decision made by a federal agency of medical experts. This attack, if allowed to stand, could have far-reaching implications weakening the authority of the FDA in matters relating to many other drugs. In April, the case was appealed to the U.S. Supreme Court, and that court ordered that the drug would remain widely available for now in a statement that did not include any reasoning as to how it might eventually rule on the case.<ref name=SupremeCourtApr23 /> It seems likely to many observers that the Supreme Court might be reluctant to overturn a medical decision by the FDA, and it might also fail to accept the "standing" of the people who filed the lawsuit in the beginning, since none of them can claim to have been harmed in any way by the drug. That the lawsuit made it as far as it did is deemed by many observers to be a function of judges overriding their legal opinion with their personal political views. As a result of the unresolved lawsuit, many states in which pregnancy termination is legal are now stockpiling the drug that is under threat of being banned. At least one state is looking into agreements for the manufacture of the drug locally within their state in the future. | In 2023, a lawsuit brought before a Texas judge (a state that is vehemently against pregnancy terminations) resulted in a ruling that would make the drug mifepristone illegal in all of the U.S. by classifying mifepristone as unsafe. The decision was immediately appealed, and stays against banning the drug were put in place. Removing mifepristone from the market would mean that medically induced abortion, the safest, most convenient and most affordable method of pregnancy termination, would be unavailable to anyone. The claim was made that the FDA had made mifepristone available without proof that it is safe, and that the court, which is not made of medical experts, should overrule a decision made by a federal agency of medical experts. This attack, if allowed to stand, could have far-reaching implications weakening the authority of the FDA in matters relating to many other drugs. In April, the case was appealed to the U.S. Supreme Court, and that court ordered that the drug would remain widely available for now in a statement that did not include any reasoning as to how it might eventually rule on the case.<ref name=SupremeCourtApr23 /> It seems likely to many observers that the Supreme Court might be reluctant to overturn a medical decision by the FDA, and it might also fail to accept the "standing" of the people who filed the lawsuit in the beginning, since none of them can claim to have been harmed in any way by the drug. That the lawsuit made it as far as it did is deemed by many observers to be a function of judges overriding their legal opinion with their personal political views. As a result of the unresolved lawsuit, many states in which pregnancy termination is legal are now stockpiling the drug that is under threat of being banned. At least one state is looking into agreements for the manufacture of the drug locally within their state in the future. | ||
On June 13, 2024, the [[Supreme Court of the United States|U.S. Supreme Court]] ruled unanimously to keep mifepristone available.<ref name=MIFE>[https://www.wsj.com/us-news/law/supreme-court-rejects-abortion-pill-challenge-preserving-wide-access-to-drug-f78f3320?st=4qzabwgst2uclm2&reflink=desktopwebshare_permalink Supreme Court Rejects Abortion Pill Challenge, Preserving Wide Access to Drug] by Laura Kusisto and Jess Bravin in the Wall Street Journal, June 13, 2024.</ref> | |||
====Oral contraceptive pills for birth control ==== | ====Oral contraceptive pills for birth control ==== | ||
Revision as of 15:06, 13 June 2024
Medical methods of contraception are forms of pregnancy prevention that include barrier contraceptives (condoms, cervical caps, etc.), birth control pills or patches (i.e, hormonal contraceptives), and IUD's (intrauterine devices). Medical contraceptive methods are all far more effective at preventing pregnancy than natural family planning.
Unlike surgical sterilization, medical methods of pregnancy prevention do not usually result in permanent sterility.
No method of birth control (medical or natural) is guaranteed to prevent all pregnancies, other than complete abstinence from sexual intercourse. Each medical method has potentially adverse physical effects, including failure to work as intended with the result being pregnancy.
Human fertility
When an egg and sperm merge, the stage is set for pregnancy. Normally, fertilization occurs in the fallopian tube where the egg will begin dividing as it travels toward the uterus, where it will become implanted. Some methods of contraception try to prevent fertilization by: (1) stopping the release of eggs (birth control pills, injections, and patches); (2) shielding the egg from sperm cells (barrier methods including condoms and diaphragms); and/or (3) impairing the sperm cells' ability to merge with the egg (spermicides in foams and sponges, copper in copper-containing IUDs). Another major strategy for contraception is to (4) prevent the implantation of a newly fertilized egg into the womb (intrauterine device (IUD)).
Because the production of gametes (the ovum and spermatazoon that are capable of uniting to produce a zygote, the very first stage of a baby) is different in women and men, the strategies for stopping their production or release by hormonal methods are also different. Women release egg cells (ovulation) periodically — usually about every 28 days, from puberty (menarche) to menopause; by contrast, men produce sperm cells (spermatogenesis) continuously after puberty. For these and other reasons, hormonal treatments to prevent production of mature gametes are only available for women at this time.
None of the medical methods of contraception, even when used in combination, is completely effective in preventing pregnancy when a woman of child-bearing age and a fertile man have sexual intercourse. Each medical contraception method has some health risks for women, and the most effective methods also pose the greatest health risk. That said, the overall risk of using the more effective methods, hormonal contraceptives (birth control pills and patches) and IUDs (once they are already implanted) is quite low, and the risk of using barrier methods such as condoms and chemical spermicides is much lower.
Sex can cause other conditions besides pregnancy
Besides the risk of pregnancy, sexual activity increases the transmission of some diseases and increased in incidence of certain conditions. Except for condoms, medical contraception methods offer little or no protection against sexually transmitted diseases, and might even increase the risk of contracting an infection. Insertion of the IUD, in particular, is associated with a high risk of pelvic inflammatory disease if a sexually transmitted infection is present when the device is inserted into the uterus. Pelvic inflammatory disease is a chronic bacterial infection of the female reproductive tract that is an important cause of infertility. Some chemical spermicides irritate the genital tract and have been shown to increase the rate of HIV transmission in both male and female partners.
Success and failure rates in contraception
Medical methods of contraception are usually described as having a particular success rate. These are summarized and tabulated by the United States Centers for Disease Control and Prevention.[1] This is the percentage of sexually active heterosexual women of childbearing age using the method over the course of a year who do not become pregnant. For example, used properly, condoms are usually described as being 98% effective. Conversely, the failure rate of contraceptives is the percentage of women who become pregnant over the course of a year despite using the method. For a hundred women whose mates used condoms, a failure rate of 2% means that two of them will become pregnant in that year. Another way that this 2% rate is expressed in the medical literature is as "two pregnancies per hundred woman-years".
A given method (such as male condoms) might be accepted as 98% effective, but real effective rates are often lower because of improper or inconsistent use of the contraceptive; for women under age 25 in the USA, male condoms are closer to 90% effective as contraceptives.
It is easy to assume that the percentage used to describe effectiveness relates to a comparison with a control group who are not using contraception. This is not accurate. When no attempt at birth control is used by sexually active couples who desire children, the rate of pregnancy is not 100%. Instead, after one year, about 85% of women will have become pregnant. The remaining couples are called infertile, and are considered candidates for medical evaluation of infertility or subfertility, should they so desire. Even without this evaluation, and without any treatment, some will go on to establish pregnancies.
Other methods (such as an IUD) have a success rate that is independent of the users' compliance. In other words, some methods have one level of effectiveness for 'perfect-use' and another level for 'actual-use', but others, such as the IUD, have an effectiveness that is completely independent of conscious motivational acts by the user.
Because these attributes are important when discussing birth control, more detailed information on effectiveness than the overall pregnancy rate is included for each of the methods presented here. Similarly, the incidence of side effects and complications of each method differ according to the individual characteristics of the women and men using them, and these relative risks are also discussed. In medicine, the choice of medical contraceptives is tailored to the individual characteristics of the user.
Clinical practice guidelines
Clinical practice guidelines are available:
Methods
Long acting methods have been reviewed.[3]
Natural family planning methods
Natural family planning, based on abstinence during the days of the month when the female is ovulating, is the least reliable form of birth control, whereby one in five women will likely become pregnant if sexually active and using only this method.[4]
Spermicides
The word 'spermicide' is usually used to refer to a product that is applied specifically for its spermicidal qualities. Spermicides are often used with, or incorporated into, other medical means of contraception. Technically, a spermicide is any material that is toxic to sperm, and, despite the "-cide" suffix, may not kill sperm so much as disable them sufficiently to halt their mobility. Some chemical spermicides are applied to condoms, or locally applied inside the vagina. However, the action of some medical contraceptives that are not ordinarily considered to be spermicides is, in fact, spermicidal. For example, the copper-containing IUDs primarily prevent fertilization by their toxic actions on sperm.
Spermicides change the cell membrane of sperm and therefore make sperm inactive. Nonoxynol-9, a widely used spermicide, can cause epithelial damage to the lining of the vagina and rectum, and this damage can increase the risk of transmission of HIV. Nonoxynol-9 is the only spermicide approved for use in the USA. "In general, when used alone, spermicides have a failure rate of approximately 15% per year with perfect use but double that rate with typical use." [5]
Since the increased risk of HIV transmission was reported by the World Health Organization in 2001, spermicides have been considered potentially problematic as contraceptives for monogomous women whose partners may be bisexual or using intravenous drugs, and for women who have many sexual partners, especially if not used in conjunction with male condoms, which offer some protection from the virus.
Barrier methods
Barrier methods try to physically prevent egg and sperm from coming into contact. Basically, this is attempted by either putting up a physical barrier to semen in the female tract, or by disabling the sperm so that it has poor mobility and a poor chance of fertilizing the egg, or both physically blocking the ejaculate and disabling the motility of sperm cells. The human sperm cell is normally able to swim, propelled by a long tail called a flagellum. When sperm cannot swim properly, they are much less likely to reach the egg. Further, when sperm are not normally motile, that is not swimming strongly, it is often a sign of being more generally impaired, and overall less likely to accomplish fertilization even if they do reach the egg.
Condoms for men
A condom is a thin, strong, non-porous sheath of material that fits over the glans and shaft of the erect penis. Historically, condoms were made from natural materials, such as sheep intestines. However, synthetic materials, such as latex, are more effective for containing semen and also offer some protection against infective agents, such as viruses, that are much smaller than sperm cells.
Condoms have become increasingly available to the public in most parts of the world since the AIDS crisis, and are the only form of contraception that (when used correctly) can reduce both the risk of pregnancy and the transmission of sexually transmitted infections. The effectiveness of condoms varies according to the individual product, and only good quality condoms are effective. Sperm cells are so small that a visible hole in a condom renders it useless. In some parts of the world, condoms are not of high enough quality to prevent sperm (or viruses) from being transmitted into the female genital tract, and these function as sham medical devices rather than true condoms. [6]
The effectiveness of a condom of good quality depends on exactly how it is used. By placing a male condom over an erect penis, semen and pre-ejaculate can be prevented from entering the vagina, stopping pregnancy from occurring; a condom can also prevent transmission of some sexually transmitted infections, including HIV, the virus that causes AIDS. When used correctly, the efficacy rate for the prevention of both pregnancy and sexually transmitted infections is 98%.[7] Water-based lubricants increase the effectiveness of condoms by reducing the chance of a tear, but oil-based products degrade latex condoms and must never be used with them latex. Polyurethane condoms or non-latex condoms are not affected by oil-based products. Even when high quality condoms containing spermicide are used appropriately, pregnancy sometimes occurs and sexually transmitted diseases, including HIV, may be transmitted.
Latex allergy
Latex allergy causes symptoms which can be very severe for people who are allergic to latex materials. Latex condoms cannot be used safely if either partner is allergic to latex.
Since the 1990s, an alternative to latex condoms has been marketed. Polyurethane condoms and synthetic elastomer condoms are not allergenic to individuals with sensitivities to latex, and, because they resist oxidation, they have a long shelf life and are compatible with oil-based lubricants. However, non-latex condoms break more easily than latex condoms and there are no good data, at present, to show whether or not they are as effective for contraception. Some non-latex condoms are not formfitting, and have been shown to be ineffective contraceptives. [8].
Effectiveness of male condoms
- Failure rates vary according to relative risk of users
While condoms, when used appropriately, are said to be 98% effective in preventing both pregnancy and the spread of sexually transmitted infections, these statistics consider all users, and not just the highest risk users. If a woman is having vaginal sexual intercourse (coitus) with a fertile man while ovulating, this 98% statistic may not apply even if the condom is used perfectly; similarly, if, over a year, a hundred HIV-negative women had intercourse solely with men who were not only HIV-positive, but who also had high concentrations of HIV antibodies and were actively shedding virus, it can not be concluded that only two of them would become HIV-positive. This 98% effectiveness rate involves the statistical realities that women sometimes have coitus when they are not fertile, and that people sometimes have sex with men who do not have any sexually transmittable diseases. For these reasons, most physicians advise discretion in choosing sexual partners for both men and women as part of the practice of 'safer sex', and awareness of the days of maximal fertility for women using barrier methods for contraception to reduce their chances of an unwanted pregnancy.
- Protection varies according to compliance: typical use is less effective than perfect use
The Effectiveness rating of male condoms has been estimated from retrospective studies in which participants were asked about their birth control methods. Although condoms are effective for contraception, and the rates quoted here have been derived from this evidence, the actual rate of effectivenesss among groups of users is not precisely known.
Male condoms, like some other barrier methods, require a deliberate act by those who use them in every instance of sexual intercourse. Further, if pregnancy and sexually transmitted disease are to be prevented, the penis cannot touch a woman's genital area unless properly covered by a condom. Using a condom, but only after such touching, or allowing such touching after the removing the condom, markedly reduces protection. For these reasons, experts caution that male condoms are most effective only when used by a motivated couple. In other circumstances, the failure rate of condoms is certainly not as low as 2%. In one study in the USA, the first-year failure rates for male condom use were between 3% and 6% when the woman was over age 30 but between 8% and 10% when the woman was under age 25.[9] According to a current obstetrics textbook, "Pregnancy rates for the condom are reported to be from 1.6 to 21 per 100 woman-years, depending on the age and motivation of the population studied." [10]
Female barriers: diaphragm, and others
Some of the female barrier methods have been in use for decades, and rates of effectiveness are fairly well known. Others, such as the female condom, are much newer and there is relatively little clinical evidence for how effective these methods are in actual use.
Condoms for women
The female condom is a loose tube of thin material (polyethelene) that extends between two rings, one which fits in the vagina and the other which fits over the external genitalia. It is not made out of latex, and therefore is not a problem for those with latex allergy. Since it completely covers the genital contact area between men and women during coitus, it should help reduce the transmission of some sexually transmitted infections, particularly genital herpes, as well as the rate of pregnancy, and — unlike the male condom, which is worn over the penis — is more completely controlled by the woman who uses it. However, the female condom is not nearly as well studied as the male condom, and the actual failure rates and effectiveness in preventing disease transmission are not known.
There are other differences which may be helpful in some circumstances: the female condom is inserted before beginning sexual activity and can be left in place for a longer time after ejaculation.
Cervical cap
The cervical cap is a flexible rubber (or plastic) cup-like dome placed over the cervix, that has been in use for several decades — mostly in Europe. A spermicide is placed in the cap before use and is an important component for effectiveness. The flexible cap is depressed while being positioned over the cervix by the user and is then held in place by suction, once released.
Cervical caps are not sold over the counter but must be carefully sized by health care providers. Caps require office or clinic visits for fitting, and personal instruction for use. Proper technique involves considerable skill on the part of the user. Even with consistent use, technical errors lead to pregnancy in 5% or more of women using this method.
Failure rates with the cervical cap are similar to those with the diaphragm. In one large, randomized clinical trial, 1-year pregnancy rates were 17.4% for the cap and 16.7% for the diaphragm.[9]
There has been speculation that the cap may help reduce sexually transmitted infections, but this device still allows the kind of genital contact that ordinarily transmits herpes simplex and genital warts, and there have been no studies that prove any reduction in sexually transmitted infections.
Diaphragm
Diaphragms are circles of synthetic material held by a flexible ring. These are folded and placed in the vagina by the user. When inserted properly, the device unfolds when released, covering the cervix. Diaphrams must be carefully fitted by a health care provider in the office or clinic in order for a woman to receive the correct size. The proper size is the largest size that fits without putting pressure on the surrounding vaginal mucosa (the lining of the vagina). Devices that are too large, or which are left in place for too long, can ulcerate the vaginal mucosa. A woman whose uterus makes a sharp angle with the vagina, or who has a short vagina, cannot usually use a diaphragm effectively.
Spermicides are recommended for use with the diaphragm. Before it is inserted, spermicial jellies or creams are placed on the side of the device that will face the cervix. The diaphragm should be left in place for at least eight hours after the last episode of sexual intercourse. If there is more than one episode, then more spermicide should be placed in the vagina before each, leaving the diaphragm in place. There are some variations in the details of recommended use depending on individual experts.
Diaphragms have been used for decades, predating the AIDS era. There is no good evidence that diaphragms prevent sexually transmitted disease. Although in some cases the diaphragm may act as a barrier that reduces infection, the use of spermicides and the possibility of ulceration with poorly fitting devices make the overall benefit doubtful. As with the cervical cap, using this device allows (essentially) full contact between the penis and vaginal mucosa, and offers no protection of surrounding areas, so there is no reason to expect protection from transmission of venereal warts or herpes. Failure rates with the diaphragm (as a contraceptive) range from about 5% for experienced, long-time users who are part of a motivated couple, to 20% for general use.[9]
Diaphragm use is associated with an increased number of urinary tract infections in the women who use them.
Intrauterine devices (IUD)
An intrauterine device (IUD) is more than 99% effective in preventing pregnancy, so that only 1 out of 100 sexually active women who use an IUD will get pregnant in a year, and if inserted within five days of unprotected sex, it still works[4]. An IUD is a 1- to 2-inch piece of flexible plastic shaped like a T that is installed by a doctor into a woman's uterus, where it remains until removed (possibly years later) and prevents implantation of a fertilized egg. An IUD can be used by a woman even if she cannot tolerate the side effects of birth control pills.
Hormonal medications (systemic)
Hormonal medications used for birth control are generally aimed at the female ovulatory cycle.[11] Since hormonal contraceptives affect the menstrual cycle, they have been prescribed for several decades for women who have problems with excessive or irregular menstrual bleeding — even if the women are celibate. More recently, some physicians have been willing to prescribe their use for elective changing of the normal menstrual cycle to decrease the number of periods. However, oral contraceptive drugs are not without significant side effects, and not all women can tolerate them. Even when women can tolerate the drug, taking it at the correct intervals requires a great deal of discipline and planning.
Systemic hormonal preparations are used for ordinary contraception in two basic forms: those that rely on progestin alone, and those that incorporate both estrogen and progestin. Ovulation is triggered in mid cycle by a surge of secretion of luteinizing hormone from the anterior pituitary gland. Progestin prevents ovulation by suppressing this secretion of luteinizing hormone. Progestins also thicken cervical mucus, thereby retarding the passage of sperm, and they make the endometrium unfavorable to implantation. Estrogen, on the other hand, prevents ovulation by suppressing the secretion of follicle-stimulating hormone (FSH), which is necessary to begin the ovarian cycle. A second effect of estrogen that is important in the contraceptives is to stabilize the endometrium, which inhibits breakthrough bleeding. Although this effect does not itself prevent pregnancy, it offers an advantage in the combination hormonal contraceptives that is not present in the progestin-only kind.
Another variation in these hormonal birth control medications is in the route of administration. If given in transdermal patches, intramuscular shots, or appliances that are inserted into the vagina, these preparations are released slowly over time (time-released) and, after administration, continue working for weeks or months. Oral contraceptives, on the other hand, are short-acting preparations that are taken daily. The difference between these medications is mainly the reduced number of acts of compliance required over a given time period in the longer-acting forms. The various preparations also have different side effects.
In some cases, antibiotics can reduce the effectiveness of oral contraception and may lead to unwanted pregnancies. In certain situations, additional contraceptive methods are recommended. [12]
'Morning-after' pill (emergency contraception)
It is possible to use hormonal oral contraceptives and IUDs for emergency contraception from just after unprotected intercourse up to 120 hours later; the sooner it is begun, the more effective it is. Combined oral contraceptives are given as an initial dose followed by a second dose 12 hours later, and these large doses sometimes have the side effect of inducing nausea and vomiting. 'Plan B' (levonorgestrel) is a two-dose, progestin-only hormone that causes much less nausea and vomiting, and is available over the counter in the USA.[13] "Current evidence suggests that single 1.5 mg dose of levonorgestrel, (or) mifepristone at doses ranging from 10 mg to 50 mg should be offered."..."Women receiving mifepristone should be warned that there may be a few days' delay in onset of menses. Emergency contraception should be started as soon as possible to obtain the highest efficacy"[14] There is generally less delay in menses when levonorgestrel is administered.
These hormones reduce the rate of pregnancy compared to controls who had unprotected sex and used no contraception, but the mechanism of that reduction is likely to vary from case to case. In some cases, ovulation may be inhibited, in others, sperm motility impaired, and in others, implantation of the fertilized egg may be stopped. However, the primary means of contraception seems to be prevention of fertilization. Dr Vivian Dickerson, then president-elect of the American College of Obstetricians and Gynecologists, stated in testimony to the FDA in December 2003: “Clinical research data demonstrate that Plan B primarily prevents pregnancy by inhibiting or preventing ovulation and secondarily perhaps by impairing the migration and function of sperm. In other words, it prevents pregnancy before fertilization occurs.”[15]
Induced abortion
Abortion has never been intended to be a method of birth control. However, it is the last resort for women whose method of birth control has failed to prevent pregnancy. From 1973 until 2022, women in the U.S. had the legal right to terminate a pregnancy within three months of impregnation, three months being a de facto standard set by the medical community. Abortions later than three months generally required doctors to certify that the woman's life would be in danger by carrying the fetus to term. But in 2022, the U.S. Supreme Court ruled, in Dobbs V. Jackson, that the question of whether abortions are legal was no longer considered a federal matter and would be left to individual states. As of 2023, around 15 states have affirmed a woman's human right to choose whether to bear a child or not, some states have yet to weigh in for or against, and in about 25 states there exist a variety of levels of bans, about half of those severe bans that may rule out abortion in cases of rape, incest, or when a woman's own life is endangered by the fetus. The point is, that induced abortion is now only legal in some states.
Two pharmaceuticals used in combination are necessary for this often-preferred medical method of terminating a pregnancy within three months of impregnation. The first drug, mifepristone, blocks the reproductive hormone progesterone, and the second, misoprostol, is taken one or two days later to prompt contractions and help the uterus expel its contents.[16] As of 2023, more than half of all pregnancies terminations in the U.S. are done using these drugs rather than by surgical methods. The Cleveland Clinic has a web page which provides a complete description of how these pharmaceuticals can be used, including procedural details, risks and benefits, recovery and outlook, and when to call the doctor.[17] The two drugs have been available for combined use since 2000, but in 2016, the U.S. Food and Drug Administration (FDA), deeming the two drugs proven safe by hundreds of medical studies and long use, took a series of steps to increase the availability of distribution of mifepristone. In 2023, this means that, in states where terminating a pregnancy is legal, people can obtain the two prescription drugs at a local drug store or by a mail-order, similar to any other medicine.[18] Women may take them at home, while remaining in touch with a nurse or doctor via phone or internet throughout the process[19], and thus avoid the risk of harrassment and threats of violence that have frequently been encountered by women who visit a birth control clinic and also, sometimes, to avoid involving family members or friends who might raise objections. As such, abortion opponents were particularly incensed that these drugs might make it easier for a woman to terminate a pregnancy without their family knowing about it.
In 2023, a lawsuit brought before a Texas judge (a state that is vehemently against pregnancy terminations) resulted in a ruling that would make the drug mifepristone illegal in all of the U.S. by classifying mifepristone as unsafe. The decision was immediately appealed, and stays against banning the drug were put in place. Removing mifepristone from the market would mean that medically induced abortion, the safest, most convenient and most affordable method of pregnancy termination, would be unavailable to anyone. The claim was made that the FDA had made mifepristone available without proof that it is safe, and that the court, which is not made of medical experts, should overrule a decision made by a federal agency of medical experts. This attack, if allowed to stand, could have far-reaching implications weakening the authority of the FDA in matters relating to many other drugs. In April, the case was appealed to the U.S. Supreme Court, and that court ordered that the drug would remain widely available for now in a statement that did not include any reasoning as to how it might eventually rule on the case.[20] It seems likely to many observers that the Supreme Court might be reluctant to overturn a medical decision by the FDA, and it might also fail to accept the "standing" of the people who filed the lawsuit in the beginning, since none of them can claim to have been harmed in any way by the drug. That the lawsuit made it as far as it did is deemed by many observers to be a function of judges overriding their legal opinion with their personal political views. As a result of the unresolved lawsuit, many states in which pregnancy termination is legal are now stockpiling the drug that is under threat of being banned. At least one state is looking into agreements for the manufacture of the drug locally within their state in the future.
On June 13, 2024, the U.S. Supreme Court ruled unanimously to keep mifepristone available.[21]
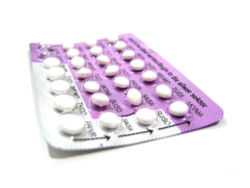
Oral contraceptive pills for birth control
With perfect use, the protection that birth control pills provide against pregnancy is nearly 100%. However, because of inconsistent or incorrect use, there is a surprisingly high annual failure rate of 8% in typical users.[22]
Pregnancy while taking these pills is almost always because of a failure to remember to take them every day (failure of compliance). Because a sudden drop in progestin can result in the release of an egg and proliferation of the uterine endometrium in preparation for implantation, missing progestagen-only pills at any time of the cycle can result in pregnancy if unprotected intercourse occurs. Missing combined pills, in particular at the beginning or at the end of the pack such that the pill-free interval is more than seven days, can also result in pregnancy.[23] Although the protection rate from unwanted pregnancy is similar in both types of pills (if used correctly), the side effects differ in incidence and type. Estrogen taken in the combined pill has a safety profile that is dose-related. A dose of 50 μg or less of the hormone has been considered safe for healthy women over age 35 years, and is also somewhat protective of bone mineralization. Estrogen administration increases the risk of thromboembolism, the creation of abnormal blood clots that can cause heart attacks, leg problems, and stroke. In women who have other risk factors for producing blood clots, such as obesity, combination birth control pills are usually not an optimal choice. Smoking, another high risk factor for thromboembolism, is generally considered to be forbidden while using oral contraceptives for this reason.
The use of oral contraceptives, including newer agents, increases blood pressure by as much as 8 mm Hg systolic and 6 mm Hg diastolic. Women with hypertension, particularly if poorly controlled, are usually advised to use a different method of contraception because of the increased risk of stroke and other adverse effects related to high blood pressure. In 2006, the American College of Obstetrics and Gynecology released guidelines suggesting that "In women with well-controlled and monitored hypertension who are 35 years or younger, a trial of combination contraceptives may be appropriate as long as the patient is otherwise healthy, shows no signs of end-organ vascular disease, and does not smoke. If blood pressure remains well controlled several months after the trial is started, combination contraceptives may be continued. Progestin-only contraceptives and the levonorgestrel-releasing intrauterine system (Mirena) are appropriate options in women with hypertension."
Combination birth control pills have been advocated as the best contraceptive for sexually active teenage girls. "As a method, these agents are the best choice for adolescents because they provide effective contraception, increase bone density, and can be used to improve acne and regulate irregular menses. The obvious disadvantage is the daily requirement of taking a pill.[24]
Oral contraceptive pills for other purposes
Oral contraceptives are sometimes prescribed for purposes other than contraception. When a woman has a condition that is treatable by oral contraceptives, then sometimes this becomes her preferred method for contraception, because of 'side benefits'. Very irregular menstruation or heavy bleeding, or dysmennorhea are some of these conditions.
Long-acting hormonal contraceptives
Long-acting hormonal contraceptives deliver the same kinds of medications as do both oral contraceptives (birth control pills) and some post-coital emergency contraceptives (such as Plan B). In the long-acting contraceptives, it is mainly the method of administration that is different from that of these short-acting preparations. Unlike oral contraceptive pills, daily doses are not needed. The advantage of long-acting contraceptives is that compliance for a period of weeks or months requires only one motivational act, whereas short-acting contraceptives require many (daily) acts by the individual using them to give the same level of protection. If each is used properly, however, the side effects of the long-acting preparations are potentially greater than those of the short-acting preparations, with some exceptions. Decrease in bone-mineral density may occur.[25]
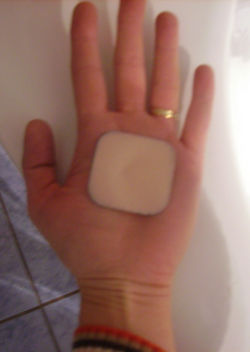
Hormonal patch
Transdermal patches are drug delivery devices that administer medication through the skin. These patches have been used to administer many different kinds of drugs, and in recent years a combination contraceptive has been formulated: Ortho Evra (Ortho McNeil). They are designed to deliver 150 µg of norelgestromin and 20 µg of ethinylestradiol every day for seven days [26]. Their primary advantage was hoped to be to eliminate the need for the daily act of compliance required to take oral contraceptives properly.
The patch is changed each week for three weeks, followed by a week with no patch. Thus the need for daily compliance is replaced by a need for weekly action by the woman using this medication. Although the 'motivational act' required with this method is reduced from daily to weekly, the patch is not any more effective than combination oral contraceptive pills in studies of actual use.
Vaginal ring
Vaginal rings are soft polymer devices that contain hormones. Basically the vaginal ring is a drug delivery system. The vaginal mucosa can absorb the medication, which is usually self-adminstered by the patient, who inserts and removes the device. Complications have included the very rare misplacement of a vaginal ring into the bladder by insertion into the urethra. Otherwise, the adverse effects are very similar to those of oral combination contraceptives.
Manufactured in the USA and France by Organon, NuvaRing is a monthly combination hormonal contraceptive that supplies a progestin (etonogestrel) and an estrogen (ethinyl estradiol). The ring is used intravaginally for 21 days followed by a seven-day hormone-free interval. When placed in the vagina, each ring releases on average 0.12 mg/day of etonogestrel and 0.015 mg/day of ethinylestradiol over a three-week period of use. [27] "The need for compliance is restricted to the day of insertion and the day of removal, although FDA labeling allows use for up to 28 days. Patients choosing the ring were given a NuvaRing starter kit, and counseled regarding the previously mentioned hormonal side-effects. Specific mention was given to side-effects unique to the ring, including expulsion (2.6%), and increased vaginal secretions (4.8%)".[28]
Like other forms of combination hormonal contraceptives for women, the vaginal ring acts primarily by preventing ovulation through suppressing the body's production of the gonadotropins that are required for the maturation of follicles in the ovary. In two large clinical trials, pregnancy rates were between one and two pregnancies per 100 women-years for women who used this vaginal ring. [29]
Intramuscular injections
Medroxyprogesterone acetate 150 mg is administered intramuscularly every three months as Depo-provera. To help ensure that the injection is not given to a pregnant women, health care providers are advised to give the first shot in the first five days of the menstrual cycle. The next injection must be given within 14 weeks, or else a pregnancy test must be performed before this next injection is given. When injections are given properly, no more than 14 weeks apart, the effectiveness is 99%. As with all prolonged administration of progestins in doses that prevent ovulation, these injections reduce serum estrogen levels, and this can lead to reductions in bone mineral density. [30][25]
Surgical methods (reproductive sterilization)
Surgical methods, also called reproductive sterilization, are the most reliable means of preventing pregnancy and are very safe. However, none of them are reliably reversible and are, therefore, chosen only by those who accept the probability that no future biological children can be conceived. These methods, include tubal ligation and vasectomy.
Ethics of using medical contraceptives
For some people, sex and pregnancy involve some of the most important aspects of adult life. Since medical contraceptives are effective in preventing pregnancy, and (to some extent) in uncoupling the 'act of procreation' from procreation itself, many ethical issues are raised by their use. At a most fundamental level, there are strongly held beliefs by some people that interfering with the establishment of pregnancy in any way other than not having sex is immoral and against the laws of God (see the article natural family planning). However, the ethical issues associated with using these methods of contraception are not restricted to religious considerations. There are many instances where sexual intercourse resulting in pregnancy is violently forced upon women against their will, or upon young women by incest or abuse from an adult male. The rights of violated women vs. the rights of an unborn fetus are not clear to everyone. When one member of a couple is dishonest about using or not using a method, in an attempt to either establish or prevent a pregnancy without the knowledge of the other, the core of their relationship may be threatened on the basis of an ethical violation. The use of contraceptives by sexually active adolescents brings up another set of ethical issues – and in some countries, even legal issues – if parental consent is not obtained. In such circumstances either withholding or providing access to contraceptives can raise ethical concerns for health care providers over their actions. Overall, the ethics of using the methods of contraception described here is too large a topic to also be included. Yet no article on medical methods of contraception would be complete without at least mentioning the fact that these issues exist.
References
- ↑ 1.0 1.1 Anonymous (2010). www.cdc.gov (English). Centers for Disease Control and Prevention. Retrieved on 2010-06-02.
- ↑ Anonymous (2009). WHO | Family planning. World Health Organization. Retrieved on 2010-06-02.
- ↑ Peterson HB, Curtis KM (2005). "Clinical practice. Long-acting methods of contraception.". N Engl J Med 353 (20): 2169-75. DOI:10.1056/NEJMcp044148. PMID 16291986. Research Blogging.
- ↑ 4.0 4.1 How Effective are IUDs from Planned Parenthood.
- ↑ Burkman RT (2007) Chapter 36: 'Contraception and family planning.' In DeCherney AH, Nathan L (eds) Current Diagnosis & Treatment Obstetrics & Gynecology 10th Edn, ISBN 9780071439008
- ↑ Sullivan TM et al. (2006) Skewed contraceptive method mix: why it happens, why it matters. J Biosocial Sci 38:501-21 PMID 16762087
- ↑ Flannigan J (2007) Promoting sexual health: practical guidance on male condom use. Nursing Standard 21:51-7 PMID 17288318
- ↑ Gallo MF et al. (2003) Non-latex versus latex male condoms for contraception. Cochrane Database Syst Rev CD003550 PMID 12804475
- ↑ 9.0 9.1 9.2 Chapter 13: 'Family planning' in Stenchever MA et al. (2001) Comprehensive Gynecology 4th Edn, Mosby, ISBN 032301402X
- ↑ 'Pregnancy prevention and birth control' in Gabbe SG et al. (2002) Obstetrics - Normal and Problem Pregnancies 4th Edn, Churchill Livingstone ISBN 0443065721
- ↑ Kaunitz AM (2008). "Clinical practice. Hormonal contraception in women of older reproductive age.". N Engl J Med 358 (12): 1262-70. DOI:10.1056/NEJMcp0708481. PMID 18354104. Research Blogging.
- ↑ Weaver, Kate and Anna Glasier, 1999. [http://phclweb.wo.utoledo.edu/4720/antibiotics-contraceptives.pdf Interaction Between Broad-Spectrum Antibiotics and the Combined Oral Contraceptive Pill]. Contraception. 59 71-78.
- ↑ Hansen LB et al. (2007) Levonorgestrel-only dosing strategies for emergency contraception. Pharmacotherapy 27:278-84 PMID 17253917
- Dirubbo NE (2006) Counsel your patients about contraceptive options. Nurse Practitioner 31:40-4 PMID 16607211
- ↑ Cheng, Cochrane reference.
- ↑ Aschenbrenner DS (2006) Over-the-counter access to emergency contraception. Am J Nursing 106:34-6 PMID 17068428
- ↑ Opponents of the right to terminate a pregnancy call the combined use of the drugs mifepristone and misoprostol "the abortion pill".
- ↑ Medical Abortion on the Cleveland Clinic website, last accessed 4/23/2023. This website provides a complete description of how pregnancies may be terminated by taking two drugs a day or two apart, along with risks and benefits, recovery and outlook, and when to call a doctor.
- ↑ In the years before 2016, a prescription for mifepristone first required an in-person doctor visit.
- ↑ Can you get abortion pills at a pharmacy now instead of directly from a nurse or doctor? And what if abortion is illegal in my state? at Planned Parenthood online, Jan. 13, 2023
- ↑ Supreme Court Ensures, for Now, Broad Access to Abortion Pill in the New York Times, April 21, 2023
- ↑ Supreme Court Rejects Abortion Pill Challenge, Preserving Wide Access to Drug by Laura Kusisto and Jess Bravin in the Wall Street Journal, June 13, 2024.
- ↑ Kaunitz AM (2005) Beyond the pill: new data and options in hormonal and intrauterine contraception. Am J Obstet Gynecol 192:998-1004 PMID 15846172
- ↑ Chapter 17: 'Fertility Control: Current Approaches and Global Aspects' in Larsen PR (2003) Williams Textbook of Endocrinology 10th Edn, Saunders & Co ISBN 0721691846
- ↑ Chapter 32: 'Contraception' in Cunningham G et al.(2005) Williams Obstetrics 22nd Edn, McGraw-Hill ISBN 0-07-141315-4
- ↑ 25.0 25.1 Lopez LM, Grimes DA, Schulz KF, Curtis KM (2011). "Steroidal contraceptives: effect on bone fractures in women.". Cochrane Database Syst Rev (7): CD006033. DOI:10.1002/14651858.CD006033.pub4. PMID 21735401. Research Blogging.
- ↑ Chapter 17: 'Fertility Control: Current Approaches and Global Aspects' in Larsen PR (2003) Williams Textbook of Endocrinology 10th Edn, Saunders & Co ISBN 0721691846
- ↑ Maheswaran AM (2006) Mosby's Drug Consult 16th Edn, ISBN 0323040624
- ↑ Victor I, Fink RA (2006) Comparing patient telephone callback rates for different hormonal birth control delivery systems. Am J Therap 13:507-12 PMID 17122531
- ↑ Maheswaran AM (2006) Mosby's Drug Consult 16th Edn, ISBN 0323040624
- ↑ Chapter 119: 'Approach to fertility control' in Goroll, AH, Mulley AG (eds) (2007) Primary Care Medicine 5th Edn, Lippincott Williams & Wilkins ISBN 078174878