Energy balance in pregnancy and lactation: Difference between revisions
imported>Matilda Thornton-Berry No edit summary |
imported>Matilda Thornton-Berry No edit summary |
||
| Line 34: | Line 34: | ||
== Changes of hormone interactions and appetite regulators during pregnancy and lactation == | == Changes of hormone interactions and appetite regulators during pregnancy and lactation == | ||
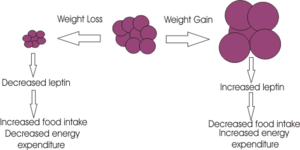
The mechanisms involved in appetite regulation during pregnancy and lactation appear to involve different neuronal pathways. Research involving hormone regulation of appetite during lactation is particularly vague. During pregnancy, changes in the expression of orexigenic neuropeptides: Neuropeptide y (NPY) and agouti-like protein (AgRP) and induced leptin resistance, contribute to increased appetite, while other mechanisms associated with offspring stimulation are thought to maintain hyperphagia during lactation (<ref> Makarvoa et al. 2008 <ref>). | |||
===Changes in orexigenic and anorectic neuropeptides during pregnacy=== | ===Changes in orexigenic and anorectic neuropeptides during pregnacy=== | ||
Research by Makarova et al. (2010) showed that both NPY and AgRP expression was increased during pregnancy and decreased after parturition. While NPY expression levels remained constant during the end of pregnancy, AgRP expression was shown to increase at the end of pregnancy, which may be responsible for constant increase in food consumption (<ref> Makarvoa et al. 2008 <ref>). During pregnancy α-MSH as no affect on decreasing food intake, hence suggesting a α-MSH resistant state is also maintained during pregnancy (<ref> Faas et al. 2009 <ref>) | |||
Studies using knock out (KO) mice of NPY had no effect on food consumption during lactation (<ref> Makarvoa et al. 2008 <ref>). Mechanisms involved in increased food consumption during lactation appear to be stimulated by the suckling action of offspring. There is an observed connection between the PVN of the hypothalamus and the nipples, which suggests that neuronal activity received by the PVN from the nipples, activates appetite stimulating neuronal pathways (<ref> Makarvoa et al. 2008 <ref>). These orexigenic neuropeptide levels are shown to increase even in presence of elevated leptin during pregnancy. | |||
===Development of leptin and insulin resistance during different reproductive states=== | ===Development of leptin and insulin resistance during different reproductive states=== | ||
=== | As previously stated, a beneficial adaptation of the female during pregnancy is to increase energy reserves which are needed to help meet the increased metabolic demands of foetal development and lactation (<ref> Faas et al. 2009). As a result of increased adiposity in the body, plasma leptin levels are elevated. Drattan et al. (2006) have shown that increased leptin levels during pregnancy are unable to suppress food intake via orexigenic neuropeptides in pregnant rats (<ref> Drattan et al. 2006 <ref>). Recorded increases in leptin levels during pregnancy is now thought to be necessary for regulating foetal growth and development (<ref> Faas et al. 2009).After partition, leptin levels decrease and leptin sensitivity is recovered. | ||
Leptin insensitivity has been shown to develop during pregnancy as studies using pregnant rats at the beginning of gestation show a decrease in food intake in response to direct leptin infusion (<ref> Ladyman et al. 2010 <ref>). Thus initial hyperphagia, induced by pregnancy, is thought to be caused by other hormonal changes which occur in the early stages of pregnancy. | |||
As obesity can be caused by leptin insensitivity and resistance within the hypothalamus, pregnancy may provide a new unique, leptin resistance model to investigate the underlying mechanisms of leptin resistance associated with obesity (<ref> Drattan et al. 2006 <ref>). | |||
As there is no evidence of a down regulation of leptin receptors in the arcuate nuclease during pregnancy (<ref> Ladyman et al. 2010 <ref>), leptin resistance may be caused by an increase in specific sex hormones during early pregnancy stages. | |||
===Roles of prolactin, progesterone and placental lactogen in leptin resistance === | |||
Increases in food intake occur very early in pregnancy when the energy demands of the foetus are still low. This observation has lead to the investigation of the roles of sex hormones in leptin resistance and hyperphagia (<ref> Faas et al. 2009). | |||
It has been suggested that progesterone stimulates appetite and food intake in pregnant females, even in the presence of leptin (<ref> Drattan et al. 2006 <ref>). | |||
Prolactin surges during early pregnancy stimulate initial orexigenic responses which are thought to include the stimulation of progesterone secretion. Placental lactogen is then thought to maintain hyperphagia by increasing leptin levels. Recorded elevated leptin levels in the pregnant female are too slow to induce leptin resistance (<ref> Drattan et al. 2006 <ref>), hence it is assumed that prolactin and placental lactogen induce leptin resistance during pregnancy, although these mechanisms of action on leptin response are unknown (<ref>Henson and Castracane 2006). | |||
Studies using pseudopregnant rats have demonstrated that chronic prolactin infusion, used to mimic placental lactogen patterns during pregnancy, inhibit the action of leptin to suppress food intake (<ref> Ladyman et al. 2010 <ref>). | |||
Revision as of 14:16, 9 November 2010
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 February 2011. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
An individual’s requirement for essential nutrients corresponds to the amount of food they consume in relation to his/her energy needs, and a woman will have very different energy metabolism and energy requirements during pregnancy . When a woman enters pregnancy she should have a consistent long-term good body size and composition, and should gain weight at a rate consistent with good health for herself and her child. The recommendations for energy intake for women vary depending on their background (population-specific) as they differ in body size and lifestyles. For example, the energy requirements are different for well-nourished women from developed countries compared to shorter women from developing countries.
Energy metabolism and energy requirements during pregnancy and lactation
Energy Metabolism
During pregnancy, women gain weight which comprises of the products of conception (foetus, placenta, amniotic fluid), the increases of various maternal tissues (uterus, breasts, blood, extracellular extravascular fluid), and the increases in maternal fat stores. Therefore the energy cost of maintenance (also known as the basal metabolic rate, BMR), as well as physical activity, increases as a result of the increased tissue mass.
This anabolic situation in pregnancy leads to a positive energy balance although some pregnant woman may also have a negative energy balance.1 This is due to the numerous metabolic adjustments that occur during pregnancy and lactation to support both foetal growth and milk synthesis without disrupting maternal homeostasis which requires retention of fat and protein in the mother and foetus. These adjustments ensure that a constant supply of glucose and amino acids reach the foetus. Adjustments also occur for lactation ensuring the mammary gland is the main area of nutrient utilization.3
INSERT FIGURE 1 FROM ENERGY METABOLISM DURING HUMAN PREGNANCY.
Energy is needed to synthesise the correct amount of fat and protein in new tissue and this consists of two components: 1. The energy in fat and protein retained in the body 2. The energy needed to synthesise these components. As it has been shown in figure 1 the total energy expenditure in pregnancy consists of four components including the energy costs for synthesizing the fat and protein retained.1
Recent information on BMR has found that the average increase during the first, second and third trimesters was 4%, 10% and 24% respectively although different women vary considerably. Women from developing countries showed a much smaller increase in BMR than those from developed countries, furthermore women with high prepregnant BMI values showed larger increases in BMR which indicates a possible increase in metabolic activity of adipose tissue in pregnancy (LINK TO JO’s SECTION). Data therefore shows that a change in BMR during pregnancy is largely a function of maternal nutritional status.
Availability of substrates to the foetus
To sustain the foetus’ growth, the mother must continuously supply it with nutrients; most importantly, glucose and amino acids. Although the placenta is almost impermeable to lipids, other than free fatty acids and ketone bodies, lipid metabolism is highly affected during pregnancy. There are two key stages during gestation; the first corresponds to the first 2/3 of the pregnancy when the foetal growth is minimal and the mother stores a great proportion of the nutrients consumed, which along with her increased food intake causes fat store accumulation. The last trimester is when the foetus grows very rapidly which is sustained through nutrient transfer through the placenta which means the mother switches to a catabolic condition. Lipid stores in particular are broken down, and glucose is the most abundant nutrient that crosses the placenta at this point.
INSERT FIGURE 2 FROM METABOLIC ADAPTATIONS IN PREGNANCY
Energy requirements
The definition of energy requirements during pregnancy can be paraphrased as "The energy requirement of a pregnant woman is the level of energy intake from food that will balance her energy expenditure when the woman has a body size and composition and level of physical activity consistent with good health, and that will allow for the maintenance of economically necessary and socially desirable physical activity. In pregnant women the energy requirement includes the energy needs associated with the deposition of tissue consistent with optimal pregnancy outcome."
Changes of hormone interactions and appetite regulators during pregnancy and lactation
The mechanisms involved in appetite regulation during pregnancy and lactation appear to involve different neuronal pathways. Research involving hormone regulation of appetite during lactation is particularly vague. During pregnancy, changes in the expression of orexigenic neuropeptides: Neuropeptide y (NPY) and agouti-like protein (AgRP) and induced leptin resistance, contribute to increased appetite, while other mechanisms associated with offspring stimulation are thought to maintain hyperphagia during lactation (Cite error: Closing </ref> missing for <ref> tag</nowiki>, like this: <ref>Person A ''et al.''(2010) The perfect reference for subpart 1 ''J Neuroendocrinol'' 36:36-52</ref> <ref>Author A, Author B (2009) Another perfect reference ''J Neuroendocrinol'' 25:262-9</ref>. Look at the reference list below to see how this will look.[5] [6] If there are more than two authors just put the first author followed by et al. (Person A at al. (2010) etc.) Select your references carefully - make sure they are cited accurately, and pay attention to the precise formatting style of the references. Your references should be available on PubMed and so will have a PubMed number. (for example PMID: 17011504) Writing this without the colon, (i.e. just writing PMID 17011504) will automatically insert a link to the abstract on PubMed (see the reference to Johnsone et al. in the list.)
[7] Use references sparingly; there's no need to reference every single point, and often a good review will cover several points. However sometimes you will need to use the same reference more than once.
How to write the same reference twice:
Reference: Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591
First time: <ref name=Berridge07>Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. ''Psychopharmacology'' 191:391–431 PMID 17072591 </ref>
Second time:<ref name=Berridge07/>
This will appear like this the first time [8] and like this the second time [8]
Figures and Diagrams
You can also insert diagrams or photographs (to Upload files Cz:Upload)). These must be your own original work - and you will therefore be the copyright holder; of course they may be based on or adapted from diagrams produced by others - in which case this must be declared clearly, and the source of the orinal idea must be cited. When you insert a figure or diagram into your article you will be asked to fill out a form in which you declare that you are the copyright holder and that you are willing to allow your work to be freely used by others - choose the "Release to the Public Domain" option when you come to that page of the form.
When you upload your file, give it a short descriptive name, like "Adipocyte.png". Then, if you type {{Image|Adipocyte.png|right|300px|}} in your article, the image will appear on the right hand side.
References
- ↑ Forsum E, Lof M (2007) Energy metabolism during human pregnancy Ann Rev Nutrition 27:277-92
- ↑ Butte NF, King JC (2005) Energy requirements during pregnancy and lactation. Public Health Nutrition 8:1010-27
- ↑ Butte NF et al. ( ) Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation Am J Clin Nutr 69:299-307
- ↑ Herrera E (2000) Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr 54:S47-S51
- ↑ Person A et al. (2010) The perfect reference for subpart 1 J Neuroendocrinol 36:36-52
- ↑ Author A, Author B (2009) Another perfect reference J Neuroendocrinol 25:262-9
- ↑ Johnstone LE et al. (2006)Neuronal activation in the hypothalamus and brainstem during feeding in rats Cell Metab 2006 4:313-21. PMID 17011504
- ↑ 8.0 8.1 Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591