Carnot cycle: Difference between revisions
imported>Paul Wormer (New page: A '''Carnot cycle''' is a reversible cycle in the state space of a thermodynamic system that has as net effect that heat is converted into work. {{Image|Carnot...) |
imported>Paul Wormer No edit summary |
||
| Line 1: | Line 1: | ||
A '''Carnot cycle''' is a reversible cycle in the state space of a [[thermodynamics|thermodynamic system]] that has as net effect that [[heat]] is converted into [[work]]. | A '''Carnot cycle''' is a reversible cycle in the state space of a [[thermodynamics|thermodynamic system]] that has as net effect that [[heat]] is converted into [[work]]. An abstract heat engine that undergoes Carnot cycles is known as a [[Carnot engine]]. | ||
{{Image|Carnot cycle.png|right| | Around 1820 [[Sadi Carnot]], while studying the efficiency of [[steam engine]]s, invented an abstract, idealized, version of steam engine with the purpose of serving as a vehicle for [[Gedankenexperimente]] (thought experiments). In 1824 he published the results of his studies in a booklet (see its title page on the left).<ref>''Reflexions on the Motive Power of Fire'', translated and edited by R. Fox, Manchester University Press, (1986) [http://books.google.nl/books?id=tVzSAAAAIAAJ&printsec=frontcover&source=gbs_v2_summary_r&cad=0#v=onepage&q=&f=false Google books]</ref> By means of this engine he tried to find answers to questions as: Would it be advantageous to use steam at high pressures, and if so, would there be a limit pressure at which it seizes to be advantageous? How important is the working medium, is steam the best substance, or could air, or other liquids, do as well? | ||
{{Image|Carnot title page.jpg|left| | |||
Roughly speaking, a steam engine consists of a piston, moving cyclically moving in and out a cylinder, that is driven by expanding steam that moves from the boiler to the condenser. | |||
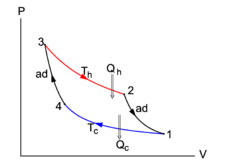
Fig. 1. consists of the isotherms 2-3 and 1-4 and the adiabats (isentropes) 1-2 and 3-4. | |||
{{Image|Carnot cycle.png|right|225px|Fig. 1. <small>The ''P-V'' diagram of a Carnot cycle. The area enclosed by the curves is the net work performed by the system (arrows clockwise)</small>}} | |||
{{Image|Carnot title page.jpg|left|275px|Reflections on the motive power of fire and on the machines fitted to develop that power.}} | |||
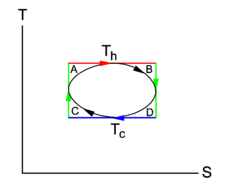
{{Image|Carnot cycle TS.png|right|225px|Fig. 2. <small>''T-S'' diagram of Carnot cycle. The green area is the net heat absorbed by the system (arrows clockwise).</small>}} | |||
'''(To be continued)''' | |||
Revision as of 07:21, 13 November 2009
A Carnot cycle is a reversible cycle in the state space of a thermodynamic system that has as net effect that heat is converted into work. An abstract heat engine that undergoes Carnot cycles is known as a Carnot engine.
Around 1820 Sadi Carnot, while studying the efficiency of steam engines, invented an abstract, idealized, version of steam engine with the purpose of serving as a vehicle for Gedankenexperimente (thought experiments). In 1824 he published the results of his studies in a booklet (see its title page on the left).[1] By means of this engine he tried to find answers to questions as: Would it be advantageous to use steam at high pressures, and if so, would there be a limit pressure at which it seizes to be advantageous? How important is the working medium, is steam the best substance, or could air, or other liquids, do as well?
Roughly speaking, a steam engine consists of a piston, moving cyclically moving in and out a cylinder, that is driven by expanding steam that moves from the boiler to the condenser.
Fig. 1. consists of the isotherms 2-3 and 1-4 and the adiabats (isentropes) 1-2 and 3-4.
(To be continued)
- ↑ Reflexions on the Motive Power of Fire, translated and edited by R. Fox, Manchester University Press, (1986) Google books