Carbodiimide: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk m (typo) |
||
| Line 3: | Line 3: | ||
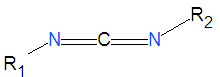
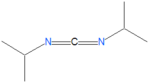
[[Image:Carbodiimide.png|right|thumb|250px|A generic carbodiimide.]] | [[Image:Carbodiimide.png|right|thumb|250px|A generic carbodiimide.]] | ||
'''Carbodiimides''' are a type of dehydrating chemical most often used to activate [[carboxylic acid]]s for subsequent coupling with primary [[amine]]s, producing an [[amide]] compound. the carboxyl group is often converted to an activated compound by forming an [[N- | '''Carbodiimides''' are a type of dehydrating chemical most often used to activate [[carboxylic acid]]s for subsequent coupling with primary [[amine]]s, producing an [[amide]] compound. the carboxyl group is often converted to an activated compound by forming an [[N-hydroxysuccinimide]] [[ester]] or other esters. | ||
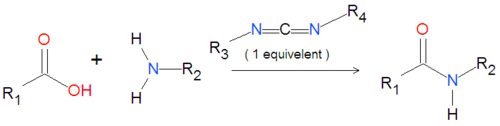
{{Image|Carbodiimide generic reaction.png|left|500px|'''Scheme 1''':Coupling of an amide group to a carboxylic acid activated by a carbodiimide.}} | {{Image|Carbodiimide generic reaction.png|left|500px|'''Scheme 1''':Coupling of an amide group to a carboxylic acid activated by a carbodiimide.}} | ||
Revision as of 12:08, 9 February 2010
Carbodiimides are a type of dehydrating chemical most often used to activate carboxylic acids for subsequent coupling with primary amines, producing an amide compound. the carboxyl group is often converted to an activated compound by forming an N-hydroxysuccinimide ester or other esters.
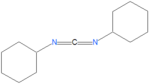
A variety of carbodiimides are commonly used, including EDAC, DIC and DCC, illustrated below. EDAC is particularly useful in aqueous reactions and is sold in a variety of biochemical reagent kits designed for coupling proteins to amines, including amines on the surface of quantum dots or nanocrystal. For solid-phase reactions, N,N'-dicyclohexylcarbodiimide (DCC) and N,N'-diisopropylcarbodiimide (DIC) are often chosen as the activating reagent.
Reaction mechanisms
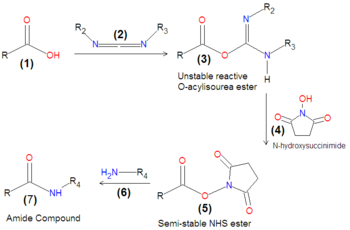
As depicted in Scheme 2, the carbodiimide-mediated coupling of a carboxylic acid (1) with a primary amine first progresses through an unstable intermediate, an O-acylisourea ester (3) compound formed by the reaction of the carbodiimide (2) with the carboxylic acid (1). Because this intermediate is unstable, it is often the case that either NHS (N-hydroxysuccinimide) 4 or sulfo-NHS is added to the carboxylic acid together with the carbodiimide, in order to form an NHS-ester (5), which is a much more stable compound that remains reactive with amines. Upon the final addition of an amine (6), the final desired coupling product, an amide (7), is formed by displacement of the NHS.
Commonly used carbodiimide reagents
EDAC
The reagent 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide, abbreviated as EDAC, EDC or EDCI, is a widely used reagent for coupling water soluble carboxylic acids with water soluble primary amides under aqueous conditions and within a pH range of 4-6.
DCC
The reagent N,N'-dicyclohexylcarbodiimide, also called 1,3-dicyclohexylcarbodiimide and abbeviated as DCC, was one of the first carbodiimides developed for amide and ester formation from carboxylic acids. It is useful for solid-phase reactions, and has been particularly useful for peptide synthesis because of its high yields for amide forming reactions and its inexpensive cost. Purification difficulties occur using this reagent, and because of this, EDC is often used preferantially to DCC.
DIC
The reagent N,N'-diisopropylcarbodiimide, also called 1,3-diisopropylcarbodiimide and abbreviated as DIC, is carbodiimide reagent often used in solid-phase synthesis preferentially over DCC because it is a liquid, and is therefore easier to use then the waxy reagent DCC. DIC is much easier to remove, by extraction techniques, compared to DCC which is otherwise very similar in reactivity.