Cefpodoxime: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>David E. Volk |
||
| Line 19: | Line 19: | ||
== Mechanism of action == | == Mechanism of action == | ||
Like other [[cephalosporin]]s and [[peniciillin]]s, cefpodoxime inhibits the proper formation of bacterial cells walls in the last stage of cell wall synthesis. Because cefpodoxime is stable against many beta-[[lactamase]]s, many organisms that are resistant to penicillins and cephalosporins, due to their beta-lactamase production, may be susceptible to cefpodoxime. Some extended-spectrum beta-lactamases may inactivate cefpodoxime. | Like other [[cephalosporin]]s and [[peniciillin]]s, cefpodoxime inhibits the proper formation of bacterial cells walls in the last stage of cell wall synthesis. Because cefpodoxime is stable against many beta-[[lactamase]]s, many organisms that are resistant to penicillins and cephalosporins, due to their beta-lactamase production, may be susceptible to cefpodoxime. Some extended-spectrum beta-lactamases may inactivate cefpodoxime. | ||
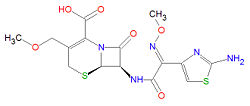
== Chemistry == | |||
The systematic IUPAC chemical name for cefpodoxime is <nowiki>(6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid</nowiki>, and its chemical formula, C<sub>15</sub>H<sub>17</sub>N<sub>5</sub>O<sub>6</sub>S<sub>2</sub>, gives is a molecular mass of 427.4554 g/mol. Like other cephalosporins and the penicillins, cefpodoxime contains a beta-[[lactam]] as the active functional group, subject to ring opening and irreversible binding via [[acylation]] to penicillin binding proteins. | |||
== Brand names == | == Brand names == | ||
Revision as of 11:49, 14 July 2008
|
| |||||||
| cefpodoxime | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | cephalosporin beta-lactam | ||||||
| Hazards: | |||||||
| |||||||
Cefpodoxime is a third-generation cephalosporin antibiotic useful against many Gram-postitive and Gram-negative bacteria, including enteric bacteria and other eubacteria. Cefpodoxime proxetil is an oral prodrug that becomes de-esterified into the cefpodoxime, the active metabolite.
Mechanism of action
Like other cephalosporins and peniciillins, cefpodoxime inhibits the proper formation of bacterial cells walls in the last stage of cell wall synthesis. Because cefpodoxime is stable against many beta-lactamases, many organisms that are resistant to penicillins and cephalosporins, due to their beta-lactamase production, may be susceptible to cefpodoxime. Some extended-spectrum beta-lactamases may inactivate cefpodoxime.
Chemistry
The systematic IUPAC chemical name for cefpodoxime is (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, and its chemical formula, C15H17N5O6S2, gives is a molecular mass of 427.4554 g/mol. Like other cephalosporins and the penicillins, cefpodoxime contains a beta-lactam as the active functional group, subject to ring opening and irreversible binding via acylation to penicillin binding proteins.
Brand names
- Vantin®
- Banan®
- Doxef®
- Orelox®
- Otreon®
- Podomexef®
The most up-to-date information about Cefpodoxime and other drugs can be found at the following sites.
- Cefpodoxime - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Cefpodoxime - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Cefpodoxime - Detailed information from DrugBank.