Count Rumford: Difference between revisions
imported>Paul Wormer m (→Biography: typos) |
imported>Todd Coles (subpages) |
||
| Line 1: | Line 1: | ||
{{subpages}} | |||
[[Image:Sir Benjamin Thompson, Count Rumford.jpg|right|thumb|266px|{{#ifexist:Template:Sir Benjamin Thompson, Count Rumford.jpg/credit|{{Sir Benjamin Thompson, Count Rumford.jpg/credit}}<br/>|}}Colonel Benjamin Thompson, FRS. Painting by Thomas Gainsborough 1783]] | [[Image:Sir Benjamin Thompson, Count Rumford.jpg|right|thumb|266px|{{#ifexist:Template:Sir Benjamin Thompson, Count Rumford.jpg/credit|{{Sir Benjamin Thompson, Count Rumford.jpg/credit}}<br/>|}}Colonel Benjamin Thompson, FRS. Painting by Thomas Gainsborough 1783]] | ||
'''Count Rumford''' was born as Benjamin Thompson on 26 March 1753 in | '''Count Rumford''' was born as Benjamin Thompson on 26 March 1753 in | ||
Revision as of 18:26, 14 March 2008
Count Rumford was born as Benjamin Thompson on 26 March 1753 in Woburn Massachusetts. He died in Auteuil near Paris on 21 August 1814. Count Rumford built up during his lifetime an international reputation as a soldier, statesman, scientist, inventor and social reformer in America, England, Bavaria and France. He was a colonel in the British Army; was elected a Fellow of the Royal Society in England in 1779; was knighted by King George III in 1781; and was made a Count of the Holy Roman Empire by the Duke of Bavaria in 1792.
Biography
Benjamin Thompson was the son of a Massachusetts farmer who died when Benjamin was twenty months old. The boy visited grade school in Woburn leaving school at the age of thirteen. After that Benjamin Thompson took care of his own education and studied chemistry, physics, and anatomy. At the age of nineteen, in November 1772, he married a rich, eleven years older, widow. His wife introduced him to the ruling circles of New England where he made such an impression that at the age of twenty he received a major's commission in the second New Hampshire Regiment, even though he had no military experience whatsoever.
Years of war of independence
During the American war of Independence (1775-1783) Major Thompson became a loyalist, that is, he chose the side of the British. While staying in Boston he did some spying on the revolutionary army. When Boston was taken by the Americans on 17 March 1776 Thompson fled to England leaving his wife and daughter behind. He would never see his wife again, but his daughter Sarah would join him in Europe twenty years later, in March 1796.
In England he befriended Lord George Germain, the Secretary of State for the Colonies in the cabinet of Lord North. Thompson provided Germain with accurate information about the progress of the American war and in return Germain gave Thompson an entry into English society. Germain's influence with King George III procured Thompson an appointment as Secretary of the Province of Georgia, which although a meaningless sinecure, brought him £100 per annum. This position left him enough time to perform an important series of scientific experiments on the properties of gunpowder. He investigated whether humid—water containing—gunpowder was more forceful than dry powder, as was often thought. He deviced a very clever experimental setup, with both the gun and its heavy wooden target as free-swinging pendulums, so that he could measure accurately the forces of recoil and impact. He found that the vaporization of water did not improve the quality of gunpowder, or in other words, dry gunpowder works better. He published his findings in a 99-page paper entitled New Experiments upon Gunpowder, in the 1781 volume of the Philosophical Transactions of the Royal Society. Its scientific worth was recognized and Thompson was made a Fellow of the Royal Society at the age of twenty-seven.
Thompson climbed rapidly in English society and in 1779 he was appointed deputy to the Inspector General of Provincial Forces. This gave Thompson sole administrative responsibility for providing clothing and other stores for the British colonial armed forces. This position enabled him to make good money. He was able to take advantage of the fact that silk, widely used in uniforms, is hygroscopic; dry silk bought in London would absorb 10 percent of its weight in the journey across the Atlantic. So, there was considerable profit to be made by selling and buying silk by weight. It was reported that Thompson made for himself about £7000 per year, but nobody seemed to mind and in 1780 he was promoted to Under Secretary of State for the Colonies.
In February 1781 he bought himself (for the sum of £4500) an appointment as lieutenant-colonel of the King's American dragoons. He left his post as Under Secretary quite unexpectedly when he sailed for New York on 7 October 1781. His ship was blown off course and he landed in Charleston almost two months later (29 December). In America the war was going very badly for the British and it took Thompson until April before he arrived in New York. Here he started to enlist men for his regiment and by August 1782 the King's American Dragoons were ready for service. However, soon after (on 30 November 1782) the hostilities ceased and peace was signed on 3 September 1783. Before that Thompson got promotion to full colonel, and after the war he retired on half-pay for the rest of his life, even though he had served in the British army only for about sixteen months.
Bavarian years
Without any specific plans Thompson traveled in the fall of 1783 to middle Europe with the intention of possibly doing a bit of soldiering. But it turned out that Europe was peaceful at that particular time and had no need of British colonels. Soon the American farmer's son befriended Prince Maximilian von Zweibrücken, which was an amazing feat given the class consciousness and the importance attached to noble birth in eighteenth century Europe. Through Maximilian's influence Thompson became colonel in the Bavarian army and aide-de-camp to the Prince-Elector (German: Kurfürst) of Bavaria, Karl II (Karl Philipp Theodor, 1724 - 1799). In those days Bavaria was an independent state within the Holy Roman Empire.
But, before Thompson could accept this position, he had to return to England to ask for permission as technically he was still a servant of King George III of Britain. The English government saw his appointment as an excellent chance to gain influence in Bavaria, and did not only grant him permission but also knighted him. Sir Benjamin was free to accept any position in Bavaria he wanted. Thompson would stay in Bavaria for twelve years climbing through the ranks. He became Minister of War with the rank of major-general, Minister of Police, Chamberlain to he Court and State Councillor. On 9 May 1792 Karl II promoted him to the rank and dignity of the Imperial Counts of the Holy Roman Empire. Sir Benjamin Thompson FRS chose the title Count Rumford, after the New Hampshire town Rumford (now Concord).
By and large Thompson deserved these honors, because he had a great impact for the good on Bavarian society, which was in a very bad shape before Thompson's arrival. It was estimated that about five percent of Bavarians lived on begging. Some gangs of beggars had Mafia-like proportions. The Bavarian army was in disarray, with about a quarter of the men being officers. Although Bavaria is completely landlocked and had no navy, it did have a great admiral. The common soldiers were mainly conscripted, unwilling, and untrained peasants. Full of energy Thompson tackled these problems. He increased the pay of the soldiers and set up schools to educate them; he established a military academy for the training of officers and he introduced scientific principles in nutrition, so that the men were fed well (and for less cost). He also reorganized the manufacturing of cannon.
With regard to the problem of the many beggars he had the simple plan to round them all up and employ them in workhouses to make military uniforms. This plan worked to a large extent since Thompson took care that the regime in the workhouses was benign and inmates were trained in a wide variety of skills and paid on a piecework basis. The social experiment was a success and many beggars were reabsorbed into society. Another great success was Thompson's construction of the "English Garden", which still exists in the city of Munich today. When it opened to the public in 1791 it was the finest park in Europe.
Politically the situation was shifting in the wrong direction for Count Rumford—as Benjamin Thompson was then called—at the end of 1792. During his energetic enterprises he had made many enemies in Munich and, moreover, his protector, Karl Theodor (Karl II), was ageing and losing power. So in March 1793 the count found it opportune to be out of sight for a while and went on holidays to Italy. Here he stayed for almost sixteen months. When Rumford returned to Bavaria in the summer of 1794, the political situation was still the same—not very favorable for the Count. While in Italy Rumford had met Alessandro Volta and the secretary of the Royal Society who happened to be in Italy also. These meetings inspired Rumford to spend more time on science. The Elector granted the Count six months leave of absence to pursue his scientific endeavors, which Rumford decided to spend in London.
Back in England
Back in England Count Rumford wrote several essays about his work in Munich on bettering the conditions of the poor. New work was his article on the improvement of the construction of chimneys. He used his understanding of convection to design a better fireplace, with a shelf at the back of the chimney so that cold air falling down the chimney struck this shelf and was deflected to join the hot air rising from the fire. This prevented clouds of smoke entering the room. In London Rumford was appreciated as a scientist and a statesman and he stretched his leave to almost a year. His daughter Sally (Sarah) joining him in March 1796, the Count felt so benevolent to mankind that he endowed two prize medals with his own money. One was to the Royal Society in London and the other to the American Academy of Arts and Sciences in Boston. They are still awarded by both institutions today.
In August 1796 Count Rumford could not postpone his return to Munich any longer because the city was in great danger. It was caught in the middle between opposing French and Austrian armies and the powers ruling Munich thought that a foreigner would be a convenient scapegoat if the town were invaded. So Rumford became Town Commandant. The Austrians set up camp on the North side of town and the French to the west. Each army was determined to occupy Munich, but Rumford, shuttling between the camps and playing for time, managed to avoid triggering any conflict until the French were pulled out following the defeat of another French army. Rumford, who had defended Munich without a drop of blood being shed, was honored by a monument in the English garden and his daughter was made Countess. When the danger was over, Rumford found time to do his famous cannon boring experiments that established the thermodynamic connection between heat and work. However, Rumford's impopularity with the ruling classes grew with the day, and it seemed better all around that Rumford would return to England. As a face-saving resolution the Elector appointed Count Rumford to Minister Plenipotentiary to the Court of St James (Bavarian Ambassador in London), where Rumford arrived 19 September 1798.
Back in London he found that King George III did not accept his credentials because as a British national he could not serve a foreign government. The King's decision turned out well for science, because Rumford found the time to establish a combined museum, research and educational institute, which was granted the Royal Seal in Januari 1800 to become the Royal Institution of Great Britain. The first professor of the Royal Institution, Thomas Garnett, was soon replaced by Humphry Davy, who was to make the institution a huge success in promoting the public understanding of the natural sciences.
Last years in Paris
Soon after appointing Davy, Rumford visited Munich to pay his respects to the successor of Elector Karl Theodor (Karl II). The new Elector was his old friend Maximilian von Zweibrücken, who took the name Maximilian IV. (Shortly before the end of the Holy Roman Empire in 1806 he became King Maximilian I of Bavaria). On his way back to England Rumford stopped over in Paris where he made the acquaintance of the widow of Lavoisier. They started an affair and in 1804 the couple settled in a house in Paris. In October 1805, after Rumford received proof that his American wife was deceased, the couple married. Although they had had an affair for almost five years, they soon found that they were incompatible and separated after a couple of years. Rumford found a house in Auteuil about four miles from the center of Paris where he lived from 1808 until his death at the age of sixty-one.
Rumford's science
Earlier, it was already mentioned that Thompson's research on the explosive power of gunpowder obtained him a membership of the Royal Society. In the second half of the 1780s, while he was restructuring the Bavarian army, Colonel Thompson's interest in army uniforms led him to the study of the conduction of heat. He discovered that convection of air is an important carrier of heat, but that still air is a good insulator. The Royal Society awarded him the Copley medal for this work. Thompson was the kind of scientist who loved to apply his findings to practical applications. In this case it lead him to the design of fluffy woollen army uniforms for the winter and cotton uniforms for the summer.
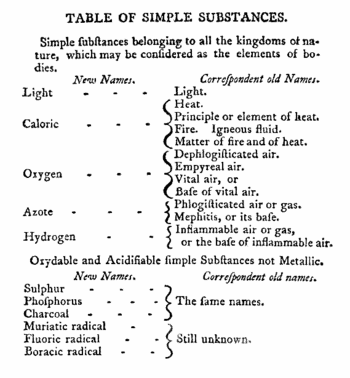
At the end of the eighteenth century there were two competing theories of heat (flow of energy from hot to cold). Antoine Lavoisier held that heat, better called caloric, is a fluid, similarly as dephlogisticated air is better called oxygen; see the reproduction of part of Lavoisier's book on the left. Another theory—now generally accepted—held that heat is a form of motion of the particles constituting matter. The particles of a hot body move more vehemently than the ones of a cold body. Count Rumford adhered to the latter theory. If heat/caloric were a fluid of which a finite amount would be contained in matter, it would be possible to exhaust it, in the same way as burning a log of wood gives off a finite amount of heat—ashes are deplete of caloric.
Rumford demonstrated in Munich around 1797, shortly after he saved the city from plunder by either the French or the Austrian army, that the heat generated by friction is inexhaustible. To show this, he bored a brass cylinder, waste product of the Munich cannon foundry, with a blunt drill bit. The setup was such that the cylinder was rotated by two horses who did their work via a system of gears, while the drill bit was stationairy. The friction generated an enormous amount of heat which Rumford was able to measure quantitatively by heating water. The conclusion was that heat was inexhaustible: as long as the horses kept working and the drill bit was boring the cylinder, heat continued being generated. Rumford could quickly bring large quantities of water to boil without any fire, to much astonishment of visitors which he showed the experiment. But Rumford liked to point out that this is not the most efficient way to heat water, more efficient would be, he said, the burning of the hay that fed the horses. With this remark he was on the edge of understanding that energy is conserved (first law of thermodynamics) and that energy can be converted from one form to another. It took about fifty more years before this principle was understood by men as Julius Mayer and James Joule.
Work is a form of energy. For instance, the work necessary to lift a kilogram from the surface of the Earth to a height of one meter is 9.81 joule (see Acceleration due to gravity). Energy used to have also a definition in terms of heat: one kilo calory (kcal) of energy can heat a liter water from 14.5 to 15.5 0C. Rumford was the first to see the equivalence of work and heat and to transform the measure of heat into the measure of work. From his cannon-boring experiments he determined that 1 cal = 5.60 joule[2]
A further blow to the caloric theory was when Rumford determined that heat is weightless (the physicist says massless). This can be expected if heat is a motion of constituting particles; if it is a fluid it can be expected to have some weight. Rumford filled three identical bottles with equal weights of water, mercury and alcohol, all of temperature 16 0C. Since on cooling down the three substances give off widely varying amounts of heat (the compounds have very different heat capacity), it could be expected that after cooling down to −1 0C, the bottles have different weight. Such a "zero experiment" can be executed with great accuracy, and this is what Rumford did, finding no weight difference whatsoever.
Rumford presented his findings in a communication entitled Enquiry Concerning the Source of Heat Which Is Excited by Friction, to the Royal Society in 1798, Since then the idea that heat is a form of motion is generally accepted.
To be continued
References
- ↑ Antoine Lavoisier Traité élémentaire de chimie, 2 vols. Chez Cuchet, Paris (1789). Translated from the French by Robert Kerr, Elements of Chemistry, 4th edition. William Creech, Edinburgh: (1790) p. 175
- ↑ Later measurements refined this number to 4.184, and we are no so certain of the equivalence of work and heat that there is only one SI unit of energy, the joule. The calory is now defined as 4.184 joule.
- S.C. Brown, Benjamin Thompson, Count Rumford, MIT Press, Cambridge, Mass., (1979)
- G.I. Brown, Scientist, Soldier, Statesman, Spy, Count Rumford, Sutton Publishing, Phoenix Mill (1999)
- D. Kleppner, About Benjamin Thompson, Physics Today, vol. 45, p. 9 (1992)
