Estrogen: Difference between revisions
imported>Robert Badgett (→Estrogen replacement therapy (ERT): Added references) |
imported>Robert Badgett m (→Estrogen replacement therapy (ERT): moved out-of-place reference) |
||
| Line 8: | Line 8: | ||
== Estrogen replacement therapy (ERT) == | == Estrogen replacement therapy (ERT) == | ||
The increase in cardiovascular mortality observed during the HERS<ref name="pmid9718051">{{cite journal |author=Hulley S, Grady D, Bush T, ''et al'' |title=Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group |journal=JAMA |volume=280 |issue=7 |pages=605–13 |year=1998 |pmid=9718051 |doi=|url=http://jama.ama-assn.org/cgi/content/full/288/3/321}}</ref> and [[Women’s Health Initiative]]<ref name="pmid12117397">{{cite journal |author=Rossouw JE, Anderson GL, Prentice RL, ''et al'' |title=Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial |journal=JAMA |volume=288 |issue=3 |pages=321–33 |year=2002 |pmid=12117397 |doi=|url=http://jama.ama-assn.org/cgi/content/full/288/3/321}}</ref> | The increase in cardiovascular mortality observed during the HERS<ref name="pmid9718051">{{cite journal |author=Hulley S, Grady D, Bush T, ''et al'' |title=Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group |journal=JAMA |volume=280 |issue=7 |pages=605–13 |year=1998 |pmid=9718051 |doi=|url=http://jama.ama-assn.org/cgi/content/full/288/3/321}}</ref> and [[Women’s Health Initiative]]<ref name="pmid12117397">{{cite journal |author=Rossouw JE, Anderson GL, Prentice RL, ''et al'' |title=Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial |journal=JAMA |volume=288 |issue=3 |pages=321–33 |year=2002 |pmid=12117397 |doi=|url=http://jama.ama-assn.org/cgi/content/full/288/3/321}}</ref><ref name="pmid15082697">{{cite journal |author=Anderson GL, Limacher M, Assaf AR, ''et al'' |title=Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial |journal=JAMA |volume=291 |issue=14 |pages=1701–12 |year=2004 |pmid=15082697 |doi=10.1001/jama.291.14.1701|url=http://jama.ama-assn.org/cgi/content/full/291/14/1701}}</ref> [[randomized controlled trial]]s has led several practicioners to cease recommending hormone replacement therapy to most of their menopausal patients.<ref name="pmid12824204">{{cite journal |author=Austin PC, Mamdani MM, Tu K, Jaakkimainen L |title=Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women's Health Initiative Study |journal=JAMA |volume=289 |issue=24 |pages=3241–2 |year=2003 |pmid=12824204 |doi=10.1001/jama.289.24.3241}}</ref> This might be, in many cases, a misinterpretation of the data. | ||
The results of the trial allow to conclude that it is possible and desirable to initiate HRT in women aged between 50 and 59 if they have vasomotor symptoms, have been menopausal for less than 10 years, are treated with statins, and have a lipid profile and a body weight that are satisfactory. This combination of factors is not rare.<ref name="pmid14645165">{{cite journal |author=Neves-E-Castro M |title=Menopause in crisis post-Women's Health Initiative? A view based on personal clinical experience |journal=Hum. Reprod. |volume=18 |issue=12 |pages=2512–8 |year=2003 |pmid=14645165 |url=http://humrep.oxfordjournals.org/cgi/content/abstract/18/12/2512}}</ref> | The results of the trial allow to conclude that it is possible and desirable to initiate HRT in women aged between 50 and 59 if they have vasomotor symptoms, have been menopausal for less than 10 years, are treated with statins, and have a lipid profile and a body weight that are satisfactory. This combination of factors is not rare.<ref name="pmid14645165">{{cite journal |author=Neves-E-Castro M |title=Menopause in crisis post-Women's Health Initiative? A view based on personal clinical experience |journal=Hum. Reprod. |volume=18 |issue=12 |pages=2512–8 |year=2003 |pmid=14645165 |url=http://humrep.oxfordjournals.org/cgi/content/abstract/18/12/2512}}</ref> | ||
Revision as of 22:34, 10 March 2008
An estrogen is a type of steroid hormone with eighteen carbons. With increasing age and menopause, the levels of estrogens decrease in women, and estrogen replacement therapy has been used for decades to decrease the systoms associated with menopause. However, recent studies suggest that estrogen replacement therapy increases the risks of heart attacks and strokes, so its use is now declining. The steroids estrone and estradiol are both estrogens.
Biosynthesis
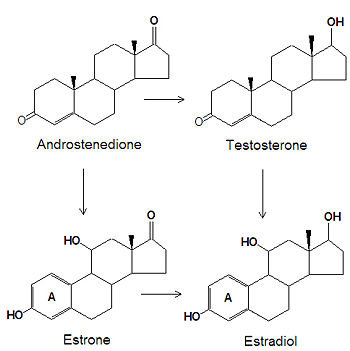
The estrogens are biosynthesized, by several chemical modifications of androgens. The C-3 ketone group is reduced to an alcohol, the C-19 methyl group is removed and the "A" ring (see steroid for numbering and nomenclature) becomes an aromatic ring.
Estrogen replacement therapy (ERT)
The increase in cardiovascular mortality observed during the HERS[1] and Women’s Health Initiative[2][3] randomized controlled trials has led several practicioners to cease recommending hormone replacement therapy to most of their menopausal patients.[4] This might be, in many cases, a misinterpretation of the data.
The results of the trial allow to conclude that it is possible and desirable to initiate HRT in women aged between 50 and 59 if they have vasomotor symptoms, have been menopausal for less than 10 years, are treated with statins, and have a lipid profile and a body weight that are satisfactory. This combination of factors is not rare.[5]
In addition, the adverse outcomes seen in the WHI trial are not necessarily direct consequences of the treatment, but can result from an exacerbation by estrogen and progestogen of other abnormalities associated with menopause, aging or other factors. Seelig & al have explained how the grossly abnormal calcium:magnesium ratio seen in most of the women who participated to the trial (and most age-matched women in the USA, for instance) likely caused the adverse events; estrogen function depends on normal tissue magnesium concentrations and calcium:magnesium ratios.[6]
Cognition
Estrogen does not improve cognitive function among patients with postmenopausal women according to a systematic review of randomized controlled trials by the Cochrane Collaboration[7]
21-hydroxylase deficiency
References
- ↑ Hulley S, Grady D, Bush T, et al (1998). "Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group". JAMA 280 (7): 605–13. PMID 9718051. [e]
- ↑ Rossouw JE, Anderson GL, Prentice RL, et al (2002). "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial". JAMA 288 (3): 321–33. PMID 12117397. [e]
- ↑ Anderson GL, Limacher M, Assaf AR, et al (2004). "Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial". JAMA 291 (14): 1701–12. DOI:10.1001/jama.291.14.1701. PMID 15082697. Research Blogging.
- ↑ Austin PC, Mamdani MM, Tu K, Jaakkimainen L (2003). "Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women's Health Initiative Study". JAMA 289 (24): 3241–2. DOI:10.1001/jama.289.24.3241. PMID 12824204. Research Blogging.
- ↑ Neves-E-Castro M (2003). "Menopause in crisis post-Women's Health Initiative? A view based on personal clinical experience". Hum. Reprod. 18 (12): 2512–8. PMID 14645165.
- ↑ Seelig MS, Altura BM, Altura BT (2004). "Benefits and risks of sex hormone replacement in postmenopausal women". J Am Coll Nutr 23 (5): 482S–496S. PMID 15466949.
- ↑ Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K (2008). "Hormone replacement therapy for cognitive function in postmenopausal women". Cochrane Database Syst Rev (1): CD003122. DOI:10.1002/14651858.CD003122.pub2. PMID 18254016. Research Blogging.
External Links
- Estrogen - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank