Antibiotic: Difference between revisions
imported>David E. Volk m (→Quinolones) |
imported>David E. Volk mNo edit summary |
||
| Line 143: | Line 143: | ||
* [[Amikacin]] | * [[Amikacin]] | ||
* [[Gentamicin]] | * [[Gentamicin]] | ||
* [[Kanamycin]] | |||
* [[Netilmicin]] | * [[Netilmicin]] | ||
* [[Streptomycin]] | * [[Streptomycin]] | ||
| Line 182: | Line 183: | ||
* [[Fosfomycin]] | * [[Fosfomycin]] | ||
* [[Hetacillin]] | * [[Hetacillin]] | ||
* [[Linezolid]] | * [[Linezolid]] | ||
* [[Loracarbef]] | * [[Loracarbef]] | ||
Revision as of 15:29, 14 July 2008
Antibiotics reduce the growth or reproduction of bacteria and are used as medications to treat bacterial infections. They interfere with the life cycle of bacteria in a number of different ways. Some antibiotics, like penicillin, interfere with cell wall synthesis, while others are reverse transcriptase inhibitors that interefere with the production of viral RNA and DNA. Other antibiotics are nucleoside analogs that get incorporated into the viral RNA or DNA and act a chain terminators.
Classes of antibiotics
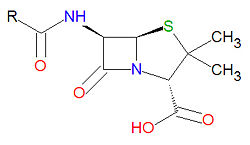
Penicillins
Penicillins have a common beta-lactam base structure, as shown, where R represents different chemical groups. Penicillins work by binding to penicillin-binding proteins irreversibly in a ring-opening reaction and disrupting bacterial cell wall synthesis. Some bacteria are resistant to penicillin because they have acquired the ability to make penicillinases, enzymes which degrade penicillin.
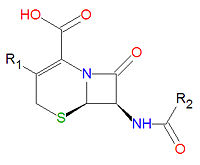
Cephalosporins
Cephalosporins are a class of antibiotic compounds sharing a common beta-lactam base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, cephalosporin C. Penicillins are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely relatedcephamycins are collectively referred to as cephems. In general, second generation and later cephalosporins have a broader spectrum of activity against Gram-negative bacteria.
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the International Nonproprietary Names (INN) suggested by the World Health Organization use the "cef" spelling for the generic drug name of all cephalosporins.
|
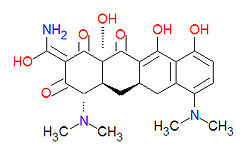
Tetracyclines
Tetracyclines are antibiotics having a common base structure consisting of four rings conjoined in a linear fashion, with differing chemical groups attached to it, typically on the bottom side or the amino group on the left side in the figure shown. Tetracyclines hinder translation by binding to the 30S ribosomal subunit and preventing the amino-acyl tRNA from binding to the A site of the ribosome, thus disrupting the synthesis of bacterial proteins.
|
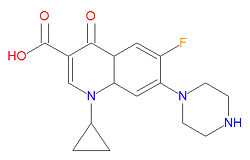
Quinolones
The mechanism of action for quinolones is different from that of macrolides, beta-lactams, aminoglycosides, or tetracyclines, so organisisms resistant to those classes of antibiotic drugs may be susceptible to quinolones. In particular, the quinolones interfere with topoisomerase enzymes, including topoisomerase II (DNA gyrase) and topoisomerase IV, which are vital to bacterial DNA replication, transcription, repair and recombination. Because the use of fluoroquinolones may lead to tendinitis or tendon rupture, especially in the Achilles tendon, the FDA requires a "black box" warning for these medications. The cause of the tendon damage is not yet determined.
Aminoglycosides
All patients taking aminoglycoside antibiotics should be under close observation due to concerns of ototoxicity and nephrotoxicity. These antibiotics have low activity against gram-positive bacteria and are often used in conjuntion with other antibiotics from a different antibiotic class. They function by inhibiting bacterial protein synthesis.
Macrolides and ketolides
Macrolide antibiotics function by binding to the 50S subunit of the bacterial 70S ribosome, thus interferring with the translocation of peptides and the production of bacterial proteins.
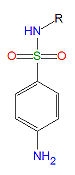
Sulfonamides
Sulfonamides are competitive inhibitors of para-aminobenzoic acid (PABA), the natural substrate for the enzymedihydropteroate synthetase, which is required within the folic acid cycle for the production of folic acid. The sulfonamides are bacteriostatic rather than bacteriocidal. Bacterial resistance to one sulfonamide indicates resistance to all of them.
Other Antibiotics (not yet classified on CZ)
- Aztreonam
- Chloramphenicol
- Clindamycin
- Colistin
- Ertapenem
- Fosfomycin
- Hetacillin
- Linezolid
- Loracarbef
- Meropenem
- Metronidazole
- Nalidixic Acid
- Neomycin
- Nitrofurantoin
- Polymyxin B Sulfate
- Procaine
- Spectinomycin
- Tinidazole
- Trimethoprim
- Vancomycin
References
Primary references