Post irradiation examination: Difference between revisions
imported>Mark Rust |
imported>Mark Rust |
||
| Line 34: | Line 34: | ||
P. Wood and G.H. Bannister published a paper on the release of <sup>85</sup>Kr, <sup>106</sup>Ru and <sup>137</sup>Cs from uranium when air is present. It was found that uranium dioxide was converted to U<sub>3</sub>O<sub>8</sub> between about 300 and 500 <sup>o</sup>C in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U<sub>3</sub>O<sub>7</sub> was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the [[krypton]]-85 was released, and that much less of the [[ruthenium]] (0.5%) and [[cesium]] (2.6 x 10<sup>-3</sup>%) occurred during the oxidation of the uranium dioxide.[http://www.iaea.org/inis/aws/htgr/fulltext/iwggcr13_11.pdf] | P. Wood and G.H. Bannister published a paper on the release of <sup>85</sup>Kr, <sup>106</sup>Ru and <sup>137</sup>Cs from uranium when air is present. It was found that uranium dioxide was converted to U<sub>3</sub>O<sub>8</sub> between about 300 and 500 <sup>o</sup>C in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U<sub>3</sub>O<sub>7</sub> was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the [[krypton]]-85 was released, and that much less of the [[ruthenium]] (0.5%) and [[cesium]] (2.6 x 10<sup>-3</sup>%) occurred during the oxidation of the uranium dioxide.[http://www.iaea.org/inis/aws/htgr/fulltext/iwggcr13_11.pdf] | ||
=== The chemical nature of fission products and the tempertures required to vapourise | === The chemical nature of fission products in uranium dioxide fuel and the tempertures required to vapourise each one === | ||
A report has been written which contains estimates of the amounts of different isotopes emitted during the chernobyl fire, the fire and explosion at chernobyl is one of the largest releases of radioactivity into the environment which has occured and hence deserves special attention([[OECD]] NEA report on Chernobyl {ten years on}[http://www.nea.fr/html/rp/chernobyl/allchernobyl.html]) | A report has been written which contains estimates of the amounts of different isotopes emitted during the chernobyl fire, the fire and explosion at chernobyl is one of the largest releases of radioactivity into the environment which has occured and hence deserves special attention([[OECD]] NEA report on Chernobyl {ten years on}[http://www.nea.fr/html/rp/chernobyl/allchernobyl.html]) | ||
Revision as of 13:34, 22 December 2006
Post Irradiation Examination (PIE) and fuel behavior is a page devoted to the behaviour of nuclear fuel in a power reactor and the way in which used fuel is studied. It is common that experimental and production fuel will be examined after use in a reactor. [1][2] [3] [4] Due to the intensely radioactive nature of the used fuel this is done in a hot cell. A combination of nondestructive and destructive methods are used.
The PIE is used to check that the fuel is both safe and effective. After major accidents the core (or what is left of it) is normally subject to PIE in order to find out what happened. One site where PIE is done is the ITU which is the EU centre for the study of highly radioactive materials.
Swelling
It is normal for fuel to swell due to thermal expansion during use. A document on the subject can be downloaded from the NASA web site.[5]
Cracking of the fuel
This is due to the fact that as the fuel expands on heating, the core of the pellet expands more than the rim. Because of the thermal stress thus formed the fuel cracks, the cracks tend to go from the centre to the edge in a star shaped pattern.
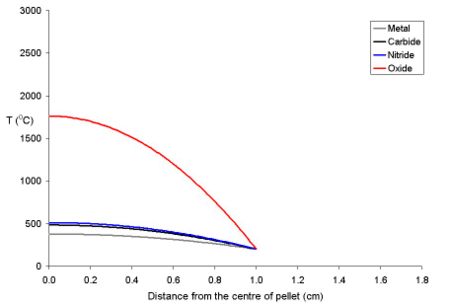
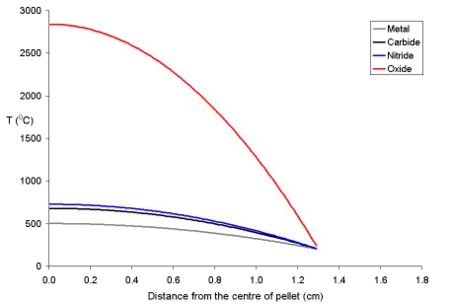
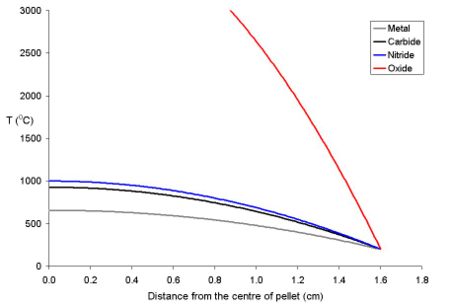
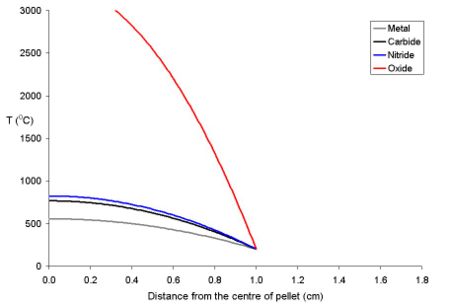
The temperature of the fuel varies as a function of the distance from the centre to the rim. At distance x from the centre the temperature (Tx) is described by the equation where ρ is the power density (W m-3) and Kf is the thermal conductivity.
- Tx = TRim + ρ (rpellet2 - x2) (4 Kf)-1
To explain this for a series of fuel pellets being used with a rim temperature of 200 oC (typical for a BWR) with different diameters and power densities of 250 Wm-3 have been modeled using the above equation. Note that these fuel pellets are rather large; it is normal to use oxide pellets which are about 10 mm in dimater.
Reference Radiochemistry and Nuclear Chemistry, G. Choppin, J-O Liljenzin and J. Rydberg, 3rd Ed, 2002, Butterworth-Heinemann, ISBN 0-7506-7463-6
Fission gas release
As the fuel is degraded or heated the more volatile fission products which are trapped within the uranium dioxide may become free. For example see J.Y. Colle, J.P. Hiernaut, D. Papaioannou, C. Ronchi, A. Sasahara, Journal of Nuclear Materials, 2006, 348, 229.
P. Wood and G.H. Bannister published a paper on the release of 85Kr, 106Ru and 137Cs from uranium when air is present. It was found that uranium dioxide was converted to U3O8 between about 300 and 500 oC in air. They report that this process requires some time to start, after the induction time the sample gains mass. The authors report that a layer of U3O7 was present on the uranium dioxide surface during this induction time. They report that 3 to 8% of the krypton-85 was released, and that much less of the ruthenium (0.5%) and cesium (2.6 x 10-3%) occurred during the oxidation of the uranium dioxide.[6]
The chemical nature of fission products in uranium dioxide fuel and the tempertures required to vapourise each one
A report has been written which contains estimates of the amounts of different isotopes emitted during the chernobyl fire, the fire and explosion at chernobyl is one of the largest releases of radioactivity into the environment which has occured and hence deserves special attention(OECD NEA report on Chernobyl {ten years on}[7])
| Element | Gas | Metal | Oxide | Solid solution | Radioisotopes | Release at Chernobyl[9] | T required for 10% release from UO2 | T required for 10% release from U3O8 |
|---|---|---|---|---|---|---|---|---|
| Br | Yes | - | - | - | - | - | - | - |
| Kr | Yes | - | - | - | 85Kr | 100% | - | - |
| Rb | Yes | - | Yes | - | - | - | - | - |
| Sr | - | - | Yes | Yes | 89Sr and 90Sr | 4-6% | 1950 K | - |
| Y | - | - | - | Yes | - | 3.5% | - | - |
| Zr | - | - | Yes | Yes | 95Zr | 3.5% | 2600 K | - |
| Nb | - | - | Yes | - | - | - | - | - |
| Mo | - | Yes | Yes | - | 99Mo | >3.5% | - | 1200 K |
| Tc | - | Yes | - | - | - | - | - | 1300 K |
| Ru | - | Yes | - | - | 103Ru and 106Ru | >3.5% | - | - |
| Rh | - | Yes | - | - | - | - | - | - |
| Pd | - | Yes | - | - | - | - | - | - |
| Ag | - | Yes | - | - | - | - | - | - |
| Cd | - | Yes | - | - | - | - | - | - |
| In | - | Yes | - | - | - | - | - | - |
| Sn | - | Yes | - | - | - | - | - | - |
| Sb | - | Yes | - | - | - | - | - | - |
| Te | Yes | Yes | Yes | Yes | 132Te | 25-60% | 1400 K | 1200 K |
| I | Yes | - | - | - | 131I | 50-60% | 1300 K | 1100 K |
| Xe | Yes | - | - | - | 133Xe | 100% | 1450 K | - |
| Cs | Yes | - | Yes | - | 134Cs and 137Cs | 20-40% | 1300 K | 1200 to 1300 K |
| Ba | - | - | Yes | Yes | 140Ba | 4-6% | 1850 K | 1300 K |
| La | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Ce | - | - | - | Yes | 141Ce and 144Ce | 3.5% | 2300 K | - |
| Pr | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Nd | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Pm | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Sm | - | - | - | Yes | - | 3.5% | 2300 K | - |
| Eu | - | - | - | Yes | - | 3.5% | 2300 K | - |
J.Y. Colle, J.-P. Hiernaut, D. Papaioannou, C. Ronchi and A. Sasahara, Journal of Nuclear Materials, 2006, 348, 229-242 reported the releases of fission products and uranium from uranium dioxide (from spent BWR fuel, burn-up was 65 GWd t-1) which was heated in a Knudsen cell. Fuel was heated in the Knudsen cell both with and without preoxidation in oxygen at c 650 K. It was found even for the noble gases that a high temperature was required to liberate them from the uranium oxide solid. For unoxidized fuel 2300 K was required to release 10% of the uranium while oxidized fuel only requires 1700 K to release 10% of the uranium.
According to the report on Chernobyl used in the above table 3.5% of the following isotopes in the core were released 239Np, 238Pu, 239Pu , 240Pu, 241Pu and 242Cm.