imported>Chunbum Park |
imported>John Stephenson |
| (145 intermediate revisions by 5 users not shown) |
| Line 1: |
Line 1: |
| == '''[[Ideal gas law]]''' ==
| | {{:{{FeaturedArticleTitle}}}} |
| ''by [[User:Milton Beychok|Milton Beychok]] and [[User:Paul Wormer|Paul Wormer]] (and [[User:Daniel Mietchen|Daniel Mietchen]] and [[User:David E. Volk|David E. Volk]])
| | <small> |
| | | ==Footnotes== |
| ----
| |
| {| class="wikitable" style="float: right;" | |
| ! Values of ''R''
| |
| ! Units
| |
| |-
| |
| | 8.314472
| |
| | [[Joule|J]]·[[Kelvin|K]]<sup>-1</sup>·[[Mole (unit)|mol]]<sup>-1</sup>
| |
| |-
| |
| | 0.082057
| |
| | [[Liter|L]]·[[atmosphere (unit)|atm]]·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 8.205745 × 10<sup>-5</sup>
| |
| | [[metre|m]]<sup>3</sup>·atm·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 8.314472
| |
| | L·k[[Pascal (unit)|Pa]]·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 8.314472
| |
| | m<sup>3</sup>·Pa·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 62.36367
| |
| | L·[[mmHg]]·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 62.36367
| |
| | L·[[torr]]·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 83.14472
| |
| | L·m[[Bar (unit)|bar]]·K<sup>-1</sup>·mol<sup>-1</sup>
| |
| |-
| |
| | 10.7316
| |
| | [[Foot (unit)|ft]]<sup>3</sup>·[[Psi (unit)|psi]]· [[Rankine scale|°R]]<sup>-1</sup>·[[lb-mol]]<sup>-1</sup>
| |
| |-
| |
| | 0.73024
| |
| | ft<sup>3</sup>·atm·°R<sup>-1</sup>·lb-mol<sup>-1</sup>
| |
| |}
| |
| | |
| The '''[[ideal gas law]]''' is the [[equation of state]] of an '''ideal gas''' (also known as a '''perfect gas''') that relates its [[Pressure#Absolute pressure versus gauge pressure|absolute pressure]] ''p'' to its [[temperature|absolute temperature]] ''T''. Further parameters that enter the equation are the [[volume]] ''V'' of the container holding the gas and the [[amount of substance|amount]] ''n'' (in [[mole (unit)|moles]]) of gas contained in there. The law reads
| |
| :<math> pV = nRT \,</math>
| |
| where ''R'' is the [[molar gas constant]], defined as the product of the [[Boltzmann constant]] ''k''<sub>B</sub> and [[Avogadro's constant]] ''N''<sub>A</sub>
| |
| :<math>
| |
| R \equiv N_\mathrm{A} k_\mathrm{B}
| |
| </math> | |
| Currently, the most accurate value of R is:<ref>[http://physics.nist.gov/cgi-bin/cuu/Value?r Molar gas constant] Obtained from the [[NIST]] website. [http://www.webcitation.org/query?url=http%3A%2F%2Fphysics.nist.gov%2Fcgi-bin%2Fcuu%2FValue%3Fr&date=2009-01-03 (Archived by WebCite® at http://www.webcitation.org/5dZ3JDcYN on Jan 3, 2009)]</ref> 8.314472 ± 0.000015 J·K<sup>-1</sup>·mol<sup>-1</sup>.
| |
| | |
| The law applies to ''ideal gases'' which are hypothetical gases that consist of [[molecules]]<ref>Atoms may be seen as mono-atomic molecules.</ref> that do not interact, i.e., that move through the container independently of each other. In contrast to what is sometimes stated (see, e.g., Ref.<ref>[http://en.wikipedia.org/w/index.php?oldid=261421829 Wikipedia: Ideal gas law] Version of January 2, 2009</ref>) an ideal gas does not necessarily consist of [[point particle]]s without internal structure, but may be formed by polyatomic molecules with internal rotational, vibrational, and electronic [[degrees of freedom]]. The ideal gas law describes the motion of the [[center of mass|centers of mass]] of the molecules and, indeed, mass centers may be seen as structureless point masses. However, for other properties of ideal gases, such as [[entropy (thermodynamics)|entropy]], the internal structure may play a role.
| |
| | |
| The ideal gas law is a useful approximation for calculating temperatures, volumes, pressures or amount of substance for many gases over a wide range of values, as long as the temperatures and pressures are far from the values where [[condensation]] or [[sublimation]] occur.
| |
| | |
| Real gases deviate from ideal gas behavior because the intermolecular attractive and repulsive forces cause the motions of the molecules to be correlated. The deviation is especially significant at low temperatures or high pressures, i.e., close to condensation. A conventional measure for this deviation is the [[Compressibility factor (gases)|compressibility factor]].
| |
| | |
| There are many equations of state available for use with real gases, the simplest of which is the [[van der Waals equation]].
| |
| | |
| === Historic background ===
| |
| | |
| The early work on the behavior of gases began in pre-industrialized [[Europe]] in the latter half of the 17th century by [[Robert Boyle]] who formulated ''[[Boyle's law]]'' in 1662 (independently confirmed by [[Edme Mariotte]] at about the same time).<ref name=Savidge>[http://www.ceesi.com/docs_techlib/events/ishm2003/Docs/1040.pdf Compressibility of Natural Gas] Jeffrey L. Savidge, 78th International School for Hydrocarbon Measurement (Class 1040), 2003. From the website of the Colorado Engineering Experiment Station, Inc. (CEESI).</ref> Their work on air at low pressures established the inverse relationship between pressure and volume, ''V'' = constant / ''p'' at constant temperature and a fixed amount of air. ''Boyle's Law'' is often referred to as the ''Boyles-Mariotte Law''.
| |
| | |
| ''[[Ideal gas law|.... (read more)]]''
| |
| | |
| {| class="wikitable collapsible collapsed" style="width: 90%; float: center; margin: 0.5em 1em 0.8em 0px;"
| |
| |-
| |
| ! style="text-align: center;" | [[Volatility (chemistry)#References|notes]]
| |
| |-
| |
| |
| |
| {{reflist|2}} | | {{reflist|2}} |
| |}
| | </small> |
After decades of failure to slow the rising global consumption of coal, oil and gas,[1] many countries have proceeded as of 2024 to reconsider nuclear power in order to lower the demand for fossil fuels.[2] Wind and solar power alone, without large-scale storage for these intermittent sources, are unlikely to meet the world's needs for reliable energy.[3][4][5] See Figures 1 and 2 on the magnitude of the world energy challenge.
Nuclear power plants that use nuclear reactors to create electricity could provide the abundant, zero-carbon, dispatchable[6] energy needed for a low-carbon future, but not by simply building more of what we already have. New innovative designs for nuclear reactors are needed to avoid the problems of the past.
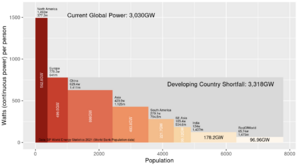
(CC) Image: Geoff Russell Fig.1 Electricity consumption may soon double, mostly from coal-fired power plants in the developing world.
[7] Issues Confronting the Nuclear Industry
New reactor designers have sought to address issues that have prevented the acceptance of nuclear power, including safety, waste management, weapons proliferation, and cost. This article will summarize the questions that have been raised and the criteria that have been established for evaluating these designs. Answers to these questions will be provided by the designers of these reactors in the articles on their designs. Further debate will be provided in the Discussion and the Debate Guide pages of those articles.
- ↑ Global Energy Growth by Our World In Data
- ↑ Public figures who have reconsidered their stance on nuclear power are listed on the External Links tab of this article.
- ↑ Pumped storage is currently the most economical way to store electricity, but it requires a large reservoir on a nearby hill or in an abandoned mine. Li-ion battery systems at $500 per KWh are not practical for utility-scale storage. See Energy Storage for a summary of other alternatives.
- ↑ Utilities that include wind and solar power in their grid must have non-intermittent generating capacity (typically fossil fuels) to handle maximum demand for several days. They can save on fuel, but the cost of the plant is the same with or without intermittent sources.
- ↑ Mark Jacobson believes that long-distance transmission lines can provide an alternative to costly storage. See the bibliography for more on this proposal and the critique by Christopher Clack.
- ↑ "Load following" is the term used by utilities, and is important when there is a lot of wind and solar on the grid. Some reactors are not able to do this.
- ↑ Fig.1.3 in Devanney "Why Nuclear Power has been a Flop"
