Sarcopenia: Difference between revisions
imported>Michael Toscano (New page: == Sarcopenia == {{TOC|right}} Sarcopenia (from Greek) literally translates as ''poverty of flesh'' <ref name=TJ> Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology [Internet]...) |
imported>Meg Taylor No edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
{{TOC|right}} | {{TOC|right}} | ||
Sarcopenia (from Greek) literally translates as ''poverty of flesh'' <ref name=TJ> Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology [Internet]. 2003 Oct [cited 2010 Sept 29]; 95(4): 1717-1727. </ref> and is used to describe a disease that results in the reduction of skeletal muscle mass and the loss of strength. In clinical studies, sarcopenia is often a qualitative inspection for small muscle mass and is widely considered one of the major causes of age-related disabilities <ref name=Lau> Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of Applied Physiology [Internet]. 2003 Nov 1 [cited 2010 Sept 29]; 95(5): 1851-1860. </ref>. Sarcopenia reflects a progressive withdrawal of [[anabolism]] and an increased [[catabolism]]. This disease also reduces the muscles ability to regenerate, due to a decrease in [ | Sarcopenia (from Greek) literally translates as ''poverty of flesh'' <ref name=TJ> Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology [Internet]. 2003 Oct [cited 2010 Sept 29]; 95(4): 1717-1727. </ref> and is used to describe a disease that results in the reduction of skeletal muscle mass and the loss of strength. In clinical studies, sarcopenia is often a qualitative inspection for small muscle mass and is widely considered one of the major causes of age-related disabilities <ref name=Lau> Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of Applied Physiology [Internet]. 2003 Nov 1 [cited 2010 Sept 29]; 95(5): 1851-1860. </ref>. Sarcopenia reflects a progressive withdrawal of [[anabolism]] and an increased [[catabolism]]. This disease also reduces the muscles ability to regenerate, due to a decrease in [[satellite cell]] numbers <ref name=Nar> Narici MV, Maffulli N. Sarcopenia: Characteristics, Mechanisms, and Functional Significance. British Medical Bulletin [Internet]. 2010 Sept [cited 2010 Sept 29]; 95(1): 139-159. </ref>. | ||
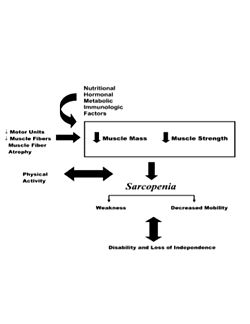
{{Image|9L5N70.jpeg|right|250px| Factors contributing to sarcopenia. This summarizes the influence of multiple things that can lead to declines in muscle mass and strength, which ultimately leads to disability and loss of independence. | {{Image|9L5N70.jpeg|right|250px| Factors contributing to sarcopenia. This summarizes the influence of multiple things that can lead to declines in muscle mass and strength, which ultimately leads to disability and loss of independence. | ||
Source: Doherty TJ. 2003. <ref name=TJ />}} | Source: Doherty TJ. 2003. <ref name=TJ />}} | ||
== Classification of Sarcopenia == | == Classification of Sarcopenia == | ||
Sarcopenic classification of individuals requires a measure that expresses muscle mass in relation to skeletal size and sex-specific criteria to determine a deficit in skeletal muscle mass. The elderly have less muscle mass and less bone mass along with expanded extracellular fluid volumes and a smaller amount of body cell mass compared to that of younger adults <ref name=Baum> Baumgartner RN, Yasumura S, Wang J, Pierson RN. Body Composition in Healthy Aging. In Vivo Body Composition Studies [Internet]. 2006 Jan 26 [cited 2010 Sept 29]; 904: 437-448. </ref>. A decrease in skeletal muscle mass is associated with a significant decline in the maximum force that can be exerted. This suggests that the quality or efficiency of skeletal muscle is reduced with age <ref name=Green> Greenlund LJS, Nair KS. Sarcopenia- consequences, mechanisms; and potential therapies. Mechanisms of Aging and Development [Internet]. 2003 Mar [cited 2010 Sept 29]; 124(3): 287-299. </ref>. | Sarcopenic classification of individuals requires a measure that expresses muscle mass in relation to skeletal size and sex-specific criteria to determine a deficit in skeletal muscle mass. The elderly have less muscle mass and less bone mass along with expanded extracellular fluid volumes and a smaller amount of body cell mass compared to that of younger adults <ref name=Baum> Baumgartner RN, Yasumura S, Wang J, Pierson RN. Body Composition in Healthy Aging. In Vivo Body Composition Studies [Internet]. 2006 Jan 26 [cited 2010 Sept 29]; 904: 437-448. </ref>. A decrease in skeletal muscle mass is associated with a significant decline in the maximum force that can be exerted. This suggests that the quality or efficiency of skeletal muscle is reduced with age <ref name=Green> Greenlund LJS, Nair KS. Sarcopenia- consequences, mechanisms; and potential therapies. Mechanisms of Aging and Development [Internet]. 2003 Mar [cited 2010 Sept 29]; 124(3): 287-299. </ref>. | ||
| Line 10: | Line 11: | ||
The loss of skeletal muscle mass directly impacts and is correlated with age-related declines in strength. Studies have shown that between the ages of 20 and 60 years old, total muscle cross section areas (CSA) decreases by about 40%. This includes a reported 25-35% CSA reduction of the quadriceps muscles in the thighs of older men and women when compared to a younger control group <ref name=TJ />. | The loss of skeletal muscle mass directly impacts and is correlated with age-related declines in strength. Studies have shown that between the ages of 20 and 60 years old, total muscle cross section areas (CSA) decreases by about 40%. This includes a reported 25-35% CSA reduction of the quadriceps muscles in the thighs of older men and women when compared to a younger control group <ref name=TJ />. | ||
== Insulin Resistance in Correlation with Sarcopenia == | == Insulin Resistance in Correlation with Sarcopenia == | ||
Loss of muscle mass associated with sarcopenia promotes insulin resistance. This creates a vicious cycle for a person’s health, leading to even further loss of muscle mass and mobility, further insulin resistance, and a risk of developing a metabolic syndrome <ref name=Nar />. This is a risk factor for the development of type II diabetes with muscle being the major metabolic organ that’s responsible for disposal of glucose and fatty acids after a meal. With reduced muscle mass, this disposal mechanism is less efficient and contributes to the cause of early hyperglycemia <ref name=Green />. | Loss of muscle mass associated with sarcopenia promotes insulin resistance. This creates a vicious cycle for a person’s health, leading to even further loss of muscle mass and mobility, further insulin resistance, and a risk of developing a metabolic syndrome <ref name=Nar />. This is a risk factor for the development of type II diabetes with muscle being the major metabolic organ that’s responsible for disposal of glucose and fatty acids after a meal. With reduced muscle mass, this disposal mechanism is less efficient and contributes to the cause of early hyperglycemia <ref name=Green />. | ||
Elderly with early sarcopenia are those who are most likely to benefit form interventions and there is strong evidence that sarcopenia is a reversible cause of disability with the use of resistance exercise and aerobics <ref name=Lau />. Studies have shown that if a person is physically inactive, that person can suffer form disuse atrophy and their muscle fibers and cells that are not exercised will begin to shrink, thus making physical inactivity a catalyst for sarcopenia. | Elderly with early sarcopenia are those who are most likely to benefit form interventions and there is strong evidence that sarcopenia is a reversible cause of disability with the use of resistance exercise and aerobics <ref name=Lau />. Studies have shown that if a person is physically inactive, that person can suffer form disuse atrophy and their muscle fibers and cells that are not exercised will begin to shrink, thus making physical inactivity a catalyst for sarcopenia. | ||
== Protein Synthesis == | == Protein Synthesis == | ||
Changes in the amount and type of skeletal protein occur as well. Resistance exercise training enhances muscle protein synthesis, improves muscle protein quality and, will thus, reverse the role of sarcopenia <ref name=Lau />. Synthesis of new structural proteins will maintain not just the skeletal muscle mass, but the muscle quality as well. Studies show that protein breakdown rates are reported to either not change at all or simply decrease with age along with mixed muscle and specific muscle proteins. Synthesis of these proteins decreases by 30% with age, as shown by Welle et al. and Balagopal et al. | Changes in the amount and type of skeletal protein occur as well. Resistance exercise training enhances muscle protein synthesis, improves muscle protein quality and, will thus, reverse the role of sarcopenia <ref name=Lau />. Synthesis of new structural proteins will maintain not just the skeletal muscle mass, but the muscle quality as well. Studies show that protein breakdown rates are reported to either not change at all or simply decrease with age along with mixed muscle and specific muscle proteins. Synthesis of these proteins decreases by 30% with age, as shown by Welle et al. and Balagopal et al. | ||
== Energy-Producing Organelles == | == Energy-Producing Organelles == | ||
Some studies believe that the loss of strength and contractile activity of muscles may be due to a decreased amount of [[mitochondria]]. The systematic formation of useable energy is crucial to the generation of contractile force including the ability to maintain repeated contractile activity. When a human performs aerobic exercise, there is a significant increase in the mitochondrial enzyme activity. Coggan et al. performed a study in which men and women of the ages 60-70 years old trained via walking or jogging at 80% maximal heart rate for 45 minutes a day, four days a week, for 9-12 months. The results: a 24-55% increase in the mitochondrial enzymes, which leads to a higher amount of energy, which leads to stronger contractile fibers in the muscles and aiding in the reversal of sarcopenia. | Some studies believe that the loss of strength and contractile activity of muscles may be due to a decreased amount of [[mitochondria]]. The systematic formation of useable energy is crucial to the generation of contractile force including the ability to maintain repeated contractile activity. When a human performs aerobic exercise, there is a significant increase in the mitochondrial enzyme activity. Coggan et al. performed a study in which men and women of the ages 60-70 years old trained via walking or jogging at 80% maximal heart rate for 45 minutes a day, four days a week, for 9-12 months. The results: a 24-55% increase in the mitochondrial enzymes, which leads to a higher amount of energy, which leads to stronger contractile fibers in the muscles and aiding in the reversal of sarcopenia. | ||
== Resistance Training == | == Resistance Training == | ||
Detecting impaired muscle function at an early stage would allow for the reversal of sarcopenia with the use of resistance exercise or training. The accumulation of intramuscular fat and connective tissue has been shown to be inversely related to the level of physical activity as one would guess. Thus, if one were to double the level of physical activity, the amount of intramuscular fat and connective tissue would literally be halved. Skeletal muscle infiltration by fat cells was not only shown to slow the movement of an individual due to the added mass that had to be carried around, but has also been shown to sustain sarcopenia due to a [[macrophage]] infiltration mediated-release of pro-inflammatory [[cytokines]] (such as TNF-α, IL-6, IL-1) and [ | Detecting impaired muscle function at an early stage would allow for the reversal of sarcopenia with the use of resistance exercise or training. The accumulation of intramuscular fat and connective tissue has been shown to be inversely related to the level of physical activity as one would guess. Thus, if one were to double the level of physical activity, the amount of intramuscular fat and connective tissue would literally be halved. Skeletal muscle infiltration by fat cells was not only shown to slow the movement of an individual due to the added mass that had to be carried around, but has also been shown to sustain sarcopenia due to a [[macrophage]] infiltration mediated-release of pro-inflammatory [[cytokines]] (such as TNF-α, IL-6, IL-1) and [[adipokines]] (leptin, adiponectin, and resistin) from [[adipocytes]]<ref name=Nar />. | ||
==Notes== | |||
{{reflist}} | |||
Latest revision as of 15:01, 14 October 2013
Sarcopenia (from Greek) literally translates as poverty of flesh [1] and is used to describe a disease that results in the reduction of skeletal muscle mass and the loss of strength. In clinical studies, sarcopenia is often a qualitative inspection for small muscle mass and is widely considered one of the major causes of age-related disabilities [2]. Sarcopenia reflects a progressive withdrawal of anabolism and an increased catabolism. This disease also reduces the muscles ability to regenerate, due to a decrease in satellite cell numbers [3].
Classification of Sarcopenia
Sarcopenic classification of individuals requires a measure that expresses muscle mass in relation to skeletal size and sex-specific criteria to determine a deficit in skeletal muscle mass. The elderly have less muscle mass and less bone mass along with expanded extracellular fluid volumes and a smaller amount of body cell mass compared to that of younger adults [4]. A decrease in skeletal muscle mass is associated with a significant decline in the maximum force that can be exerted. This suggests that the quality or efficiency of skeletal muscle is reduced with age [5].
In earlier studies, sarcopenia was examined in individuals by observing and testing calf muscle [2], and other muscle cross sectional areas for change in muscle function with aging and how it affected mobility in men and women. Also tested were the participants’ knee extension, handgrip strength, plantar flexor and dorsiflexor muscles of the foot [1], and lower extremity muscle power. Sarcopenia is considered to be present when the measurement of each of these indicators was greater than two standard deviations below the mean of the experimental group’s data.
The loss of skeletal muscle mass directly impacts and is correlated with age-related declines in strength. Studies have shown that between the ages of 20 and 60 years old, total muscle cross section areas (CSA) decreases by about 40%. This includes a reported 25-35% CSA reduction of the quadriceps muscles in the thighs of older men and women when compared to a younger control group [1].
Insulin Resistance in Correlation with Sarcopenia
Loss of muscle mass associated with sarcopenia promotes insulin resistance. This creates a vicious cycle for a person’s health, leading to even further loss of muscle mass and mobility, further insulin resistance, and a risk of developing a metabolic syndrome [3]. This is a risk factor for the development of type II diabetes with muscle being the major metabolic organ that’s responsible for disposal of glucose and fatty acids after a meal. With reduced muscle mass, this disposal mechanism is less efficient and contributes to the cause of early hyperglycemia [5].
Elderly with early sarcopenia are those who are most likely to benefit form interventions and there is strong evidence that sarcopenia is a reversible cause of disability with the use of resistance exercise and aerobics [2]. Studies have shown that if a person is physically inactive, that person can suffer form disuse atrophy and their muscle fibers and cells that are not exercised will begin to shrink, thus making physical inactivity a catalyst for sarcopenia.
Protein Synthesis
Changes in the amount and type of skeletal protein occur as well. Resistance exercise training enhances muscle protein synthesis, improves muscle protein quality and, will thus, reverse the role of sarcopenia [2]. Synthesis of new structural proteins will maintain not just the skeletal muscle mass, but the muscle quality as well. Studies show that protein breakdown rates are reported to either not change at all or simply decrease with age along with mixed muscle and specific muscle proteins. Synthesis of these proteins decreases by 30% with age, as shown by Welle et al. and Balagopal et al.
Energy-Producing Organelles
Some studies believe that the loss of strength and contractile activity of muscles may be due to a decreased amount of mitochondria. The systematic formation of useable energy is crucial to the generation of contractile force including the ability to maintain repeated contractile activity. When a human performs aerobic exercise, there is a significant increase in the mitochondrial enzyme activity. Coggan et al. performed a study in which men and women of the ages 60-70 years old trained via walking or jogging at 80% maximal heart rate for 45 minutes a day, four days a week, for 9-12 months. The results: a 24-55% increase in the mitochondrial enzymes, which leads to a higher amount of energy, which leads to stronger contractile fibers in the muscles and aiding in the reversal of sarcopenia.
Resistance Training
Detecting impaired muscle function at an early stage would allow for the reversal of sarcopenia with the use of resistance exercise or training. The accumulation of intramuscular fat and connective tissue has been shown to be inversely related to the level of physical activity as one would guess. Thus, if one were to double the level of physical activity, the amount of intramuscular fat and connective tissue would literally be halved. Skeletal muscle infiltration by fat cells was not only shown to slow the movement of an individual due to the added mass that had to be carried around, but has also been shown to sustain sarcopenia due to a macrophage infiltration mediated-release of pro-inflammatory cytokines (such as TNF-α, IL-6, IL-1) and adipokines (leptin, adiponectin, and resistin) from adipocytes[3].
Notes
- ↑ 1.0 1.1 1.2 1.3 Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology [Internet]. 2003 Oct [cited 2010 Sept 29]; 95(4): 1717-1727.
- ↑ 2.0 2.1 2.2 2.3 Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of Applied Physiology [Internet]. 2003 Nov 1 [cited 2010 Sept 29]; 95(5): 1851-1860.
- ↑ 3.0 3.1 3.2 Narici MV, Maffulli N. Sarcopenia: Characteristics, Mechanisms, and Functional Significance. British Medical Bulletin [Internet]. 2010 Sept [cited 2010 Sept 29]; 95(1): 139-159.
- ↑ Baumgartner RN, Yasumura S, Wang J, Pierson RN. Body Composition in Healthy Aging. In Vivo Body Composition Studies [Internet]. 2006 Jan 26 [cited 2010 Sept 29]; 904: 437-448.
- ↑ 5.0 5.1 Greenlund LJS, Nair KS. Sarcopenia- consequences, mechanisms; and potential therapies. Mechanisms of Aging and Development [Internet]. 2003 Mar [cited 2010 Sept 29]; 124(3): 287-299.