Human iron metabolism: Difference between revisions
imported>Howard C. Berkowitz No edit summary |
mNo edit summary |
||
| (19 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC| | {{TOC|left}} | ||
'''Iron''' is an essential nutrient for human beings, although it can also be toxic. The most important role is in the [[heme]] protein of [[hemoglobin]] and [[cytochrome P-450]], and secondarily for [[myoglobin]], which transports oxygen into muscle cells. Iron also is a component of a number of enzymes. | [[Image:Human Iron V2.png|thumb|right|400px|High-level review]] | ||
'''Iron''' is an essential nutrient for human beings, although it can also be toxic. The most important role is in the [[heme]] protein of [[hemoglobin]] and [[cytochrome P-450]], and secondarily for [[myoglobin]], which transports oxygen into muscle cells. Iron also is a component of a number of enzymes. The greatest demand for iron is from [[erythropoesis]]: hemoglobin for the production of new [[erythrocyte]]s (i.e., red blood cells). <ref name=Munoz2009>{{citation | |||
| journal = World J Gastroenterol | year = 2009 | volume = 15 | issue = 37 | pages = 4617–4626. | |||
| doi= 10.3748/wjg.15.4617. | |||
| PMCID= PMC2754509 | |||
| title = An update on iron physiology | |||
| author = Munoz M, Villar I, Garcia-Erce JA | |||
| url = http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754509/?tool=pubmed}}</ref> | |||
==Requirements== | ==Requirements== | ||
The actual requirement for iron in food is quite low, as | The actual requirement for iron in food is quite low, as the body conserves iron stores. Erythropoiesis requires 20-30 mg/day, so, with efficient conservation, only 1-2 mg/day of dietary iron is needed. <ref name=Munoz2009 /> | ||
Not all iron-containing foods, however, provide bioavailable iron. While [[spinach]] is legendary as an iron source, the iron in raw spinach is bound into a non-absorbable [[oxalic acid|oxalate]]. Cooking the spinach does make the iron bioavailable; the cartoon character [[Popeye the Sailor Man]] was correct in eating his spinach from a can. | |||
==Regulation and homeostasis== | |||
Control mechanisms for iron metabolism are not fully understood, but two models, which may coexist, form current thinking: crypt programming and hepcidin. | |||
===Crypt programming=== | |||
The principle of this model is that that enterocytes, in the crypt cells of the duodenum, take up iron from plasma via transferrin receptors 1 and 2 (TfR1 and TfR2). The more iron these cells take up from the plasma, the less they accept from the digestive tract. | |||
===Hepcidin=== | |||
A peptide hormone produced in the liver, [[hepcidin]], has received the most attention as the control mechanism. It also affects enterocytes, but also macrophages and the liver. Originally thought to be an antibacterial substance, <ref>{{citation | |||
| title = Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver | |||
| author = Park CH ''et al.'' | |||
| doi= 10.1074/jbc.M008922200 | year = 2001 | journal = Journal of Biological Chemistry | volume = 276 | pages = 7806-7810 | |||
| url = http://www.jbc.org/content/276/11/7806.long}}</ref> it is now believed to be an inhibitor of iron intake into the body, <ref name=Ganz2005>{{citation | |||
| journal = Am J Physiol Gastrointest Liver Physiol | volume = 290 | pages = G199–G203 | year = 2005 | |||
| doi = 10.1152/ajpgi.00412.2005 | |||
| url = http://ajpgi.physiology.org/cgi/content/full/290/2/G199 | |||
| title = Iron imports. IV. Hepcidin and regulation of body iron metabolism | |||
| author = Ganz T, Nemeth E | |||
}}</ref> by binding to and inactivating [[ferroportin]], according to an August 2008 paper in ''Cell Metabolism''.<ref name=eScience>{{citation | |||
| url = http://esciencenews.com/articles/2008/08/05/key.site.iron.metabolism.aids.diagnosing.anemia.chronic.disease | |||
| title = Key site in iron metabolism aids in diagnosing anemia of chronic disease | |||
| 5 August 2008 | |||
| journal = eScience | |||
| quote = summary of paper in August 2008 issue of ''Cell Metabolism'', by Jerry Kaplan, ''et al.''}}</ref> | |||
===Erythropoetin=== | |||
Besides its role in erythropoesis, erythropoetin decreases DMT1 and increases ferroportin, thus reducing iron retention in macrophages, at least in rat models. <ref name=Kong2008>{{citation | |||
| journal =J Cell Biochem | |||
| date = 2008 May 15 | volume = 104 | issue = 2 | pages = 629-41. | |||
| title = Decreased DMT1 and increased ferroportin 1 expression is the mechanisms of reduced iron retention in macrophages by erythropoietin in rats | |||
| author = Kong WN, ''et al.'' | |||
| url = http://www.ncbi.nlm.nih.gov/pubmed/18189270}}</ref> | |||
==Digestion== | ==Digestion== | ||
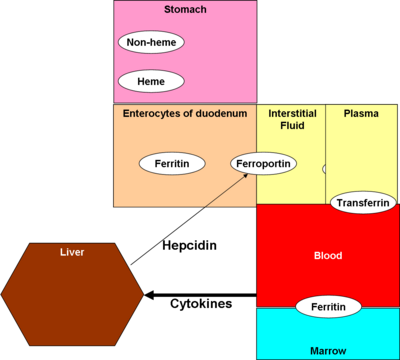
Iron, in food, is processed differently depending if it is in the form of heme (e.g., in meats) or in other molecules. As food breaks down in the [[stomach]], ferric (Fe<sup>+3</sup>) iron is reduced to ferrous (Fe<sup>+2</sup>) iron by the enzyme [[ferric reductase]]. [[Ascorbic acid]] | Iron, in food, is processed differently depending if it is in the form of heme (e.g., in meats) or in other molecules. As food breaks down in the [[stomach]], ferric (Fe<sup>+3</sup>) iron is reduced to ferrous (Fe<sup>+2</sup>) iron by the enzyme [[ferric reductase]]. [[Ascorbic acid]] and other reducing agents stimulates the reduction, and thus stimulates absorption. | ||
Most actual absorption takes place in the [[duodenum]]. Heme is absorbed via the [[heme transporter]] protein. Non-heme iron enters the apical surface of the [[enterocyte]]s lining the duodenum via [[divalent metal transporter 1]] (DMT1)<ref>{{citation | Most actual absorption takes place in the [[duodenum]]. Heme is absorbed via the [[heme transporter]] protein. Non-heme iron enters the apical surface of the [[enterocyte]]s lining the duodenum via [[divalent metal transporter 1]] (DMT1)<ref>{{citation | ||
| Line 17: | Line 55: | ||
| author = Chong WS ''et al.'' | | author = Chong WS ''et al.'' | ||
| doi=10.1093/humrep/dei246 | | doi=10.1093/humrep/dei246 | ||
| title = Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues}}</ref>, carrying iron into the [[interstitial fluid]] by a protein called [[ferroportin]] (FP). | | title = Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues}}</ref>, carrying iron into the [[interstitial fluid]] by a protein called [[ferroportin]] (FP). <ref name=Embo>{{citation | ||
| journal = EMBO Journal | year = 2006| volume = 25 | pages = 5396 - 5404 | |||
|doi= 10.1038/sj.emboj.7601409 | |||
| title = Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome | |||
| author = De Domenico, I ''et al.'' | |||
| url = http://www.nature.com/emboj/journal/v25/n22/abs/7601409a.html}}</ref> | |||
Hepcidin binds to ferroportin, inhibiting its action. <ref name=Ganz2005 /> | |||
Complete fermentation of [[phytate]]s, a longer process than is used in commercial breadmaking, increases the bioavailability of iron in cereal grains. <ref>{{citation | |||
| title = Iron Absorption from Bread in Humans: Inhibiting Effects of Cereal Fiber, Phytate and Inositol Phosphates with Different Numbers of Phosphate Groups | |||
| author = Mats Brune, Lena Rossander-Hultén, Leif Hallberg, Ann Gleerup and Ann-Sofie Sandberg | |||
| url = http://jn.nutrition.org/cgi/reprint/122/3/442 | |||
| journal = J. Nutrition | year = 1992 | |||
| volume = 122 | issue = 3 | page = 442 | |||
}}</ref> | |||
==Distribution== | ==Distribution== | ||
Transferrin moves iron through the plasma; ferritin stores it. | |||
Ferrous iron, in plasma, is reconverted to ferric, and bound to a carrier protein, [[ferritin]]. | Ferrous iron, in plasma, is reconverted to ferric, and bound to a carrier protein, [[ferritin]]. | ||
== | |||
Ferritin in plasma stays in equilibrium with ferritin in [[bone marrow]], and plasma ferritin is indicative of actual iron stores in the marrow. | |||
Marrow erythroblasts and liver hepatocytes store iron, primarily as ferritin complex but, to a lesser extent, as the breakdown product, [[hemosiderin]]. Hemosiderin is primarily present in macrophages. | |||
==Recycling and excretion== | |||
Most iron is recycled, principally by [[macrophage]]s in the [[reticuloendothelial system]]. | |||
In mouse models, hepcidin may decrease the expression of ferroportin, controlled by the FPN1 gene. <ref name=Kong2008 /> | |||
==Disorders of iron metabolism== | ==Disorders of iron metabolism== | ||
Not all [[anemia]] is due to disorders of iron metabolism, but [[iron deficiency anemia]] is a syndrome commonly seen, with many causes. Iron in excess through a metabolic error is less common, and called [[hemochromatosis]]. Among children in the U.S., iron supplements are the most common source of serious accidental poisoning. | |||
Iron loading increases hepcidin synthesis (i.e., reducing iron intake), while hypoxia and anemia decrease its production. <ref name=Ganz2005 /> | |||
Hepcidin is markedly induced during inflammation, through the effect of [[interleukin|interleukin-6 (IL-6)]]. Increased hepcidin traps iron in [[macrophage]]s, decreases plasma iron concentrations, and is a mechanism of [[anemia of chronic disease]]. <ref name=Nemeth2004>{{citation | |||
| journal = J Clin Invest | year = 2004 | volume = 113 | issue = 9 | pages = 1271–1276. | |||
| doi = 10.1172/JCI200420945. | |||
| PMCID= PMC398432 | |||
| title = IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin | |||
| author = Nemeth E ''et al'' | |||
| url = http://www.ncbi.nlm.nih.gov/pmc/articles/PMC398432/}}</ref> | |||
==References== | ==References== | ||
{{reflist}} | {{reflist|2}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 30 August 2024
Iron is an essential nutrient for human beings, although it can also be toxic. The most important role is in the heme protein of hemoglobin and cytochrome P-450, and secondarily for myoglobin, which transports oxygen into muscle cells. Iron also is a component of a number of enzymes. The greatest demand for iron is from erythropoesis: hemoglobin for the production of new erythrocytes (i.e., red blood cells). [1]
Requirements
The actual requirement for iron in food is quite low, as the body conserves iron stores. Erythropoiesis requires 20-30 mg/day, so, with efficient conservation, only 1-2 mg/day of dietary iron is needed. [1]
Not all iron-containing foods, however, provide bioavailable iron. While spinach is legendary as an iron source, the iron in raw spinach is bound into a non-absorbable oxalate. Cooking the spinach does make the iron bioavailable; the cartoon character Popeye the Sailor Man was correct in eating his spinach from a can.
Regulation and homeostasis
Control mechanisms for iron metabolism are not fully understood, but two models, which may coexist, form current thinking: crypt programming and hepcidin.
Crypt programming
The principle of this model is that that enterocytes, in the crypt cells of the duodenum, take up iron from plasma via transferrin receptors 1 and 2 (TfR1 and TfR2). The more iron these cells take up from the plasma, the less they accept from the digestive tract.
Hepcidin
A peptide hormone produced in the liver, hepcidin, has received the most attention as the control mechanism. It also affects enterocytes, but also macrophages and the liver. Originally thought to be an antibacterial substance, [2] it is now believed to be an inhibitor of iron intake into the body, [3] by binding to and inactivating ferroportin, according to an August 2008 paper in Cell Metabolism.[4]
Erythropoetin
Besides its role in erythropoesis, erythropoetin decreases DMT1 and increases ferroportin, thus reducing iron retention in macrophages, at least in rat models. [5]
Digestion
Iron, in food, is processed differently depending if it is in the form of heme (e.g., in meats) or in other molecules. As food breaks down in the stomach, ferric (Fe+3) iron is reduced to ferrous (Fe+2) iron by the enzyme ferric reductase. Ascorbic acid and other reducing agents stimulates the reduction, and thus stimulates absorption.
Most actual absorption takes place in the duodenum. Heme is absorbed via the heme transporter protein. Non-heme iron enters the apical surface of the enterocytes lining the duodenum via divalent metal transporter 1 (DMT1)[6], carrying iron into the interstitial fluid by a protein called ferroportin (FP). [7]
Hepcidin binds to ferroportin, inhibiting its action. [3]
Complete fermentation of phytates, a longer process than is used in commercial breadmaking, increases the bioavailability of iron in cereal grains. [8]
Distribution
Transferrin moves iron through the plasma; ferritin stores it.
Ferrous iron, in plasma, is reconverted to ferric, and bound to a carrier protein, ferritin.
Ferritin in plasma stays in equilibrium with ferritin in bone marrow, and plasma ferritin is indicative of actual iron stores in the marrow.
Marrow erythroblasts and liver hepatocytes store iron, primarily as ferritin complex but, to a lesser extent, as the breakdown product, hemosiderin. Hemosiderin is primarily present in macrophages.
Recycling and excretion
Most iron is recycled, principally by macrophages in the reticuloendothelial system.
In mouse models, hepcidin may decrease the expression of ferroportin, controlled by the FPN1 gene. [5]
Disorders of iron metabolism
Not all anemia is due to disorders of iron metabolism, but iron deficiency anemia is a syndrome commonly seen, with many causes. Iron in excess through a metabolic error is less common, and called hemochromatosis. Among children in the U.S., iron supplements are the most common source of serious accidental poisoning.
Iron loading increases hepcidin synthesis (i.e., reducing iron intake), while hypoxia and anemia decrease its production. [3]
Hepcidin is markedly induced during inflammation, through the effect of interleukin-6 (IL-6). Increased hepcidin traps iron in macrophages, decreases plasma iron concentrations, and is a mechanism of anemia of chronic disease. [9]
References
- ↑ 1.0 1.1 Munoz M, Villar I, Garcia-Erce JA (2009), "An update on iron physiology", World J Gastroenterol 15 (37): 4617–4626., DOI:10.3748/wjg.15.4617.
- ↑ Park CH et al. (2001), "Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver", Journal of Biological Chemistry 276: 7806-7810, DOI:10.1074/jbc.M008922200
- ↑ 3.0 3.1 3.2 Ganz T, Nemeth E (2005), "Iron imports. IV. Hepcidin and regulation of body iron metabolism", Am J Physiol Gastrointest Liver Physiol 290: G199–G203, DOI:10.1152/ajpgi.00412.2005
- ↑ "Key site in iron metabolism aids in diagnosing anemia of chronic disease", eScience
- ↑ 5.0 5.1 Kong WN, et al. (2008 May 15), "Decreased DMT1 and increased ferroportin 1 expression is the mechanisms of reduced iron retention in macrophages by erythropoietin in rats", J Cell Biochem 104 (2): 629-41.
- ↑ Chong WS et al. (Advance Access originally published online on August 25, 2005), "Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues", Human Reproduction 20 (12): 3532-3538, DOI:10.1093/humrep/dei246
- ↑ De Domenico, I et al. (2006), "Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome", EMBO Journal 25: 5396 - 5404, DOI:10.1038/sj.emboj.7601409
- ↑ Mats Brune, Lena Rossander-Hultén, Leif Hallberg, Ann Gleerup and Ann-Sofie Sandberg (1992), "Iron Absorption from Bread in Humans: Inhibiting Effects of Cereal Fiber, Phytate and Inositol Phosphates with Different Numbers of Phosphate Groups", J. Nutrition 122 (3): 442
- ↑ Nemeth E et al (2004), "IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin", J Clin Invest 113 (9): 1271–1276., DOI:10.1172/JCI200420945.