Rivaroxaban: Difference between revisions
imported>Howard C. Berkowitz No edit summary |
mNo edit summary |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{ | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:rivaroxaban.png|center|thumb|350px]] | |||
|width=350px | |||
|molname=rivaroxaban | |||
|synonyms= Xarelto | |||
|molformula= C<sub>19</sub>H<sub>18</sub>ClN<sub>3</sub>O<sub>5</sub>S | |||
|molmass= 435.88132 | |||
|uses=prevention of deep vein thrombosis | |||
|properties= anticoagulant | |||
|hazards=see side effects & drug interactions | |||
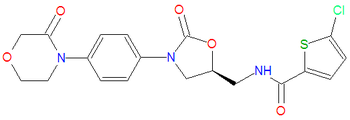
|iupac= (S)-5-chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide | |||
|casnumber= 366789-02-8 | |||
}} | |||
{{main|Coagulation}} | {{main|Coagulation}} | ||
In [[medicine]], '''rivaroxaban''' is an [[anticoagulant]] that inhibits factor Xa.<ref>{{MeSH}}</ref> Like [[warfarin]], rivaroxaban is given orgally, but unlike [[warfarin]] rivaroxaban is administered in fixed doses without the need for coagulation monitoring. | In [[medicine]], '''rivaroxaban''' is an [[anticoagulant]] that inhibits factor Xa.<ref>{{MeSH}}</ref> Like [[warfarin]], rivaroxaban is given orgally, but unlike [[warfarin]] rivaroxaban is administered in fixed doses without the need for coagulation monitoring. | ||
{{TOC|left}} | |||
==History== | ==History== | ||
Rivaroxaban was approved for use by the [http://www.emea.europa.eu European Medicines Agency] in 2009 "to prevent venous thromboembolism (VTE, the formation of clots in the veins) in adults who are undergoing surgery to replace a hip or knee."<ref>Anonymous (2009) [http://www.emea.europa.eu/humandocs/Humans/EPAR/xarelto/xarelto.htm EPARs for authorised medicinal products for human use] European Medicines Agency</ref> | Rivaroxaban was approved for use by the [http://www.emea.europa.eu European Medicines Agency] in 2009 "to prevent venous thromboembolism (VTE, the formation of clots in the veins) in adults who are undergoing surgery to replace a hip or knee."<ref>Anonymous (2009) [http://www.emea.europa.eu/humandocs/Humans/EPAR/xarelto/xarelto.htm EPARs for authorised medicinal products for human use] European Medicines Agency</ref> | ||
Rivaroxaban | Rivaroxaban was approved by the [[Food and Drug Administration]] in the [[United States of America]] 7/2011.<ref>Anonymous (2011) [http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm261839.htm FDA approves Xarelto to reduce risk of blood clots after hip, knee replacements]</ref> for "the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing knee or hip replacement surgery." | ||
==Pharmacology== | ==Pharmacology== | ||
| Line 24: | Line 44: | ||
==Clinical uses== | ==Clinical uses== | ||
===Deep venous thrombosis=== | ===Deep venous thrombosis=== | ||
Rivaroxaban can treat symptomatic [[embolism and thrombosis]] of the deep leg veins.<ref name="pmid21128814">{{cite journal| author=EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H et al.| title=Oral rivaroxaban for symptomatic venous thromboembolism. | journal=N Engl J Med | year= 2010 | volume= 363 | issue= 26 | pages= 2499-510 | pmid=21128814 | doi=10.1056/NEJMoa1007903 | pmc= | url= }} </ref> | |||
Rivaroxaban can prevent [[embolism and thrombosis]] during [[perioperative care]] according to [[randomized controlled trial]]s with two weeks of therapy after knee arthoplasty<ref name="pmid18579812">{{cite journal |author=Lassen MR, Ageno W, Borris LC, ''et al'' |title=Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty |journal=The New England journal of medicine |volume=358 |issue=26 |pages=2776–86 |year=2008 |month=June |pmid=18579812 |doi=10.1056/NEJMoa076016 |url=http://content.nejm.org/cgi/content/full/358/26/2776 |issn=}}</ref> or 5 weeks of therapy after hip arthroplasty.<ref name="pmid18579811">{{cite journal |author=Eriksson BI, Borris LC, Friedman RJ, ''et al'' |title=Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty |journal=The New England journal of medicine |volume=358 |issue=26 |pages=2765–75 |year=2008 |month=June |pmid=18579811 |doi=10.1056/NEJMoa0800374 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18579811&promo=ONFLNS19 |issn=}}</ref><ref name="pmid18582928">{{cite journal |author=Kakkar AK, Brenner B, Dahl OE, ''et al'' |title=Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial |journal=Lancet |volume=372 |issue=9632 |pages=31–9 |year=2008 |month=July |pmid=18582928 |doi=10.1016/S0140-6736(08)60880-6 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(08)60880-6 |issn=}}</ref> | Rivaroxaban can prevent [[embolism and thrombosis]] during [[perioperative care]] according to [[randomized controlled trial]]s with two weeks of therapy after knee arthoplasty<ref name="pmid18579812">{{cite journal |author=Lassen MR, Ageno W, Borris LC, ''et al'' |title=Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty |journal=The New England journal of medicine |volume=358 |issue=26 |pages=2776–86 |year=2008 |month=June |pmid=18579812 |doi=10.1056/NEJMoa076016 |url=http://content.nejm.org/cgi/content/full/358/26/2776 |issn=}}</ref> or 5 weeks of therapy after hip arthroplasty.<ref name="pmid18579811">{{cite journal |author=Eriksson BI, Borris LC, Friedman RJ, ''et al'' |title=Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty |journal=The New England journal of medicine |volume=358 |issue=26 |pages=2765–75 |year=2008 |month=June |pmid=18579811 |doi=10.1056/NEJMoa0800374 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18579811&promo=ONFLNS19 |issn=}}</ref><ref name="pmid18582928">{{cite journal |author=Kakkar AK, Brenner B, Dahl OE, ''et al'' |title=Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial |journal=Lancet |volume=372 |issue=9632 |pages=31–9 |year=2008 |month=July |pmid=18582928 |doi=10.1016/S0140-6736(08)60880-6 |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(08)60880-6 |issn=}}</ref> | ||
| Line 30: | Line 52: | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 12 October 2024
|
| |||||||
| rivaroxaban | |||||||
| |||||||
| Uses: | prevention of deep vein thrombosis | ||||||
| Properties: | anticoagulant | ||||||
| Hazards: | see side effects & drug interactions | ||||||
| |||||||
In medicine, rivaroxaban is an anticoagulant that inhibits factor Xa.[1] Like warfarin, rivaroxaban is given orgally, but unlike warfarin rivaroxaban is administered in fixed doses without the need for coagulation monitoring.
History
Rivaroxaban was approved for use by the European Medicines Agency in 2009 "to prevent venous thromboembolism (VTE, the formation of clots in the veins) in adults who are undergoing surgery to replace a hip or knee."[2]
Rivaroxaban was approved by the Food and Drug Administration in the United States of America 7/2011.[3] for "the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing knee or hip replacement surgery."
Pharmacology
Administration
Rivaroxaban is given orally
Distribution
Metabolism
Excretion
Toxicity
Clinical uses
Deep venous thrombosis
Rivaroxaban can treat symptomatic embolism and thrombosis of the deep leg veins.[4]
Rivaroxaban can prevent embolism and thrombosis during perioperative care according to randomized controlled trials with two weeks of therapy after knee arthoplasty[5] or 5 weeks of therapy after hip arthroplasty.[6][7]
External links
The most up-to-date information about Rivaroxaban and other drugs can be found at the following sites.
- Rivaroxaban - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Rivaroxaban - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Rivaroxaban - Detailed information from DrugBank.
References
- ↑ Anonymous (2024), Rivaroxaban (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Anonymous (2009) EPARs for authorised medicinal products for human use European Medicines Agency
- ↑ Anonymous (2011) FDA approves Xarelto to reduce risk of blood clots after hip, knee replacements
- ↑ EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H et al. (2010). "Oral rivaroxaban for symptomatic venous thromboembolism.". N Engl J Med 363 (26): 2499-510. DOI:10.1056/NEJMoa1007903. PMID 21128814. Research Blogging.
- ↑ Lassen MR, Ageno W, Borris LC, et al (June 2008). "Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty". The New England journal of medicine 358 (26): 2776–86. DOI:10.1056/NEJMoa076016. PMID 18579812. Research Blogging.
- ↑ Eriksson BI, Borris LC, Friedman RJ, et al (June 2008). "Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty". The New England journal of medicine 358 (26): 2765–75. DOI:10.1056/NEJMoa0800374. PMID 18579811. Research Blogging.
- ↑ Kakkar AK, Brenner B, Dahl OE, et al (July 2008). "Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial". Lancet 372 (9632): 31–9. DOI:10.1016/S0140-6736(08)60880-6. PMID 18582928. Research Blogging.